Home » Nitrogen (Page 4)
Category Archives: Nitrogen
Components of a variable rate nitrogen recomendation
I recently wrote a article for the Crops and Soils magazine on the components of a Variable Rate Nitrogen Recommendation. The people at the American Society of Agronomy headquarters were kind enough to make it open access. What follows in this blog is just a highlight reel. For the full article visit https://dl.sciencesocieties.org/publications/cns/articles/49/6/24
Components of a variable rate nitrogen recommendation
Variable-rate nitrogen management (VRN) is a fairly hot topic right now. The outcome of VRN promises improved efficiencies, economics, yields, and environmental sustainability. As the scientific community learns more about the crop’s response to fertilizer nitrogen and the soil’s ability to provide nitrogen, the complexity of providing VRN recommendations, which both maximize profitability and minimize environmental risk, becomes more evident.
The components of nitrogen fertilizer recommendations are the same whether it is for a field flat rate or a variable-rate map. The basis for all N recommendations can be traced back to the Stanford equation (Stanford, 1973). At first glance, the Stanford equation is very basic and fairly elegant with only three variables in the equation.

Historically, this was accomplished on a field level through yield goal estimates and soil test nitrate values. The generalized conversions such as 1.2 lb N/bu of corn and 2.0 lb N/bu of winter wheat took account for Ncrop and efert to simplify the process.
NCrop
The basis for Ncrop is grain yield × grain N concentration. As grain N is fairly consistent, the goal of VRN methods is to identify grain yield. This is achieved through yield monitor data, remote sensing and crop models.
NSoil
The N provided by, or in some cases removed by, the soil is dynamic and often weather dependent. Kindred et al. (2014) documented the amount of N supplied by the soil varied spatially by 107, 67, and 54 lb/ac across three studies. Much of the soil N concentration is controlled by OM. For every 1% OM in the top 6 inches of the soil profile, there is approximately 1,000 lb N/ac.
efert
Historically, the efficiency at which N fertilizer is utilized was integrated into N recommendations and not provided as an input option, e.g., the general conversion factor for corn of 1.2 lb N/bu. Nitrogen concentration in corn grain ranges from 1.23–1.46% with an average of 1.31% (Heckman et al., 2003) or 0.73 lb N/bu. Therefore, the 1.2-lb value is assuming a 60% fertilizer use efficiency. More recently, recommendations have been to incorporate application method or timing factors in attempt to account for efficiencies.
Summary
While a VRN strategy that works across all regions, landscapes, and cropping systems has yet to be developed, the process of nitrogen management has greatly improved and is evolving almost daily. Those methods that are capable of determining the three inputs of the Stanford equation while incorporating regional specificity will capture the greatest level of accuracy and precision. Ferguson et al. (2002) suggested that improved recommendation algorithms may often need to be combined with methods (such as remote sensing) to detect crop N status at early, critical growth stages followed by carefully timed, spatially adjusted supplemental fertilization to achieve optimum N use efficiency. As information and data are gathered and incorporated and data-processing systems improve in both capacity and speed, the likelihood of significantly increasing nitrogen use efficiency for the benefit of the society and industry improves. The goal of all practitioners is to improve upon the efficiencies and economics of the system, and this should be kept in mind as new techniques and methods are evaluated. This improvement can be as small as a few percentages
This article is published in the Crops and Soils Magazine doi:10.2134/cs2016-49-0609. The full article includes more details on the components plus concepts of integration.
Precision Nutrient Management in Forage Systems
Published in Progressive Forage http://www.progressiveforage.com/ 9.1.2016
First, let’s agree the term “precision” is relative. Forage is a diverse system with an even more diverse set of management strategies. Regardless, every manager should be constantly striving to improve the precision in which nutrients are managed. The ultimate goal of any precision nutrient management tool should be this: producing the highest quality output (in this case forage) with the least amount of input – ultimately, optimizing efficiencies and maximizing profits. Within this readership there are those who are soil sampling at a 1-acre resolution and others who have likely not pulled a soil sample in the past decade. For both spectrums we can make improvements – let’s start basic and move forward.
A soil sample should the basis for all nutrient management decisions. Is soil testing a perfected science? No, far from it. However, there must be a starting point. A soil sample is that first bit of information we can start with and the basic data collection for precision ag to make improved management decisions. When fertilizer is applied without a recent soil sample, it is done based upon pure guesswork. How many other management decisions are made on a farm or ranch by a guess?
The composite soil sample is a great start, but it is just that – a start. While there are some soils that are very uniform most are extremely variable. In a survey of 178 fields in the southern Great Plains on average the soil pH was 6.12; phosphorus (Mehlich 3 phosphorus [M3P] and Bray 1 phosphorus [B1P]) was 28 ppm while soil test potassium averaged 196 ppm. So on the average the primary components of soil fertility were okay. However, on average the 178 fields had a range in soil pH of 1.8 units, M3P and B1P both had range of a 52 ppm and STK had a range of 180 ppm.
Table 1 shows the minimum and maximum soil test values for the 178 fields.
| Average | Range | Min | Max | |
| Soil pH | 6.12 | 1.77 | 5.23 | 7.01 |
| Phosphorus | 28 | 52 | 2 | 54 |
| Potassium | 197 | 180 | 107 | 287 |
| Sulfur | 15 | 24 | 3 | 27 |
| Organic matter | 1.9 | 1.2 | 1.3 | 2.5 |
This data helps support the concept that we should find ways to increase the resolution or decrease the number of acres represented by a single soil sample. Increasing soil sample resolution is typically done using one or two methods – zone or by grid.
Zone sampling
The basis of a zone sample is creating a smaller field. The biggest question with zones is how to draw the lines. There are dozens, if not hundreds, of possible methods, each having their own reasons and benefits. My basic recommendation is that before lines are drawn goals have to be established. For example, if phosphorus or soil pH management is important, the basis for the lines should be soil based. This could be based on soils map, soil texture, slope and on and on. If the target is improved nitrogen management, then the reason for drawing lines should be yield based. This could be based on yield maps, aerial images, historic knowledge or many soil parameters.
Why does it matter? Two reasons: First, across the broad spectrum of soils and environments two nutrients are hardly spatially correlated, which means the zone that is best at describing phosphorus variability does an extremely poor job describing potassium variability. Second, more theoretically the demand for nutrients are driven by different factors. Phosphorus (a soil immobile nutrient) fertilizer need is driven by the soil P concentration (look up Brays Sufficiency Concept). Many use yield as a parameter for phosphorus application, but this is not a plant need or even a yield maximizing practice. Fertilizing based on removal is done to prevent nutrient mining. However, nitrogen (a nutrient mobile in the soil) fertilizer need is based on yield and crop removal. Hence, the common Land Grant University N and sulfur recommendations are yield goal based.
Grid sampling
To be honest even the experts disagree on the hows, whys and ifs of grid sampling. I like data, therefore I naturally lean towards grid sampling if the field warrants it. For me, the biggest benefit of grid over zone sampling is that soils data from zone samples are biased to whatever parameter was set for the zone and therefore any resulting map for all nutrients must reflect the original zones. In a grid, each data point is independent therefore the maps of each nutrient can be independent, and (the science tells us) in most cases nutrients are independent of each other.
Ideally two pieces of information are available for determining whether a field is grid sampled or not. The first piece of information is a yield map from any previous crop. If yield is fairly uniform, I question the need for variable rate management, much less the expense of grid sampling. Regardless of the sampling method zone or grid, the discussion is moot if spatial variability does not exist across the field. However, many forage producers may not have access to this kind of data.
One of the most useful decision aid tools for grid sampling is the composite soil sample. The reason is simple statistics: A composite sample should be a representative average of the field. If the data is normally distributed, that means half of the field is above and half the field is below the sample average. So the optimum fields to grid are those in which an input falls at the point in which the benefit of applying is in question, because it suggests that approximately half the field needs the inputs while the other half likely does not. It is in this scenario that the return on investment can be greatest. As with pH, for example, fields with a very low value should have a flat broadcast application and should be sampled again at a later date. Fields with a composite pH well above 6.0 will unlikely have enough acres needing lime to warrant sending out an applicator.
Is grid sampling a lifelong activity? No. The initial activity of grid sampling will provide both an indicator of the variability level and overall needs of the field. From that point, decisions can be made and actions taken. Identify the greatest limiting factor in the field based on the samples, and focus on impacting change upon it. Zone sampling in subsequent years can be utilized to document change. When that issue is resolved, move to the next factor. It may require grid sampling again or using the original grid to develop new management zones. For instance, if the greatest issue first identified on the field is soil acidity then after the soil pH is neutralized the field should be grid sampled again. The reason is for this is that changing soil pH will influence many nutrients and the amount of change is not consistent but dependent upon many other factors.
In precision ag we tend to look at layers, yield, soil, etc. However, none of these tell the whole story independently. An area in a field may have moderate soil fertility and be under producing. Using the data collected the decision may be made to increase inputs; yet, the issue is a shallow restrictive layer limiting production. Therefore, the extra inputs will be of no benefit and could even further reduce production. It is at this point I like to bring out the importance of “getting dirty.” There is no technology that can take the place of “boots on the ground” agronomy.
For producers who have historically preformed intensive soil sampling there is still room for improvement. Soil testing and nutrient management is not an exact science; in fact, it was originally built for broad sweeping, statewide recommendations. As technology advances and inputs can be applied at sub-acre resolutions, all of the environment (weather, soil) by genotype inactions becomes more evident.
The next step in precision ag is to develop recommendations by upon site specific crop responses. This is where nutrient response strips can further improve nutrient use efficiencies and crop production. In Oklahoma, nitrogen-rich strips are applied across fields (grain and forage) to determine in-season nitrogen needs. Having a strip in the field with 50 to 100 extra units of N acts as a management tool which takes into account soil, environment and plant need. If the strip is visible the field or zone needs more N, if it is not visible then the crop is not deficient and at that point in the season does not need more N. Producers have taken this approach for N and adopted it for P and K with strips across the field with a zero and high rate of either nutrient. After a few seasons, responsive and non-responsive zones are developed and P and K applications are managed accordingly.
One misconception of precision ag is that the end result should be a field with uniform yield from one corner to the other. This is often not the case; in fact, in many cases the variability in production across the field can be increased. Theoretically, precision ag is applying inputs at the right rate in the right place. This means areas of the field which are yield limited due to underlying factors which cannot be managed have a reduction in inputs with no effect on yield. Other areas of the field have not been managed for maximum production therefore an increase inputs result in increasing yield widening the gap between the low and high yield levels.
Regardless of where a producer currently sets on the technology curve, there are potential ways to increase productivity and efficiency. There is nothing wrong with taking baby steps; it is often the simple things that lead to the greatest return.
Planting Wheat After Anhydrous
Every year in August and early September I get the question “How soon after applying NH3 can I sow wheat?”. Typically my answer has been a conservative one which takes into account rate, depth, spacing and soil moisture to end up with a range of 3 days to a week. The concern with anhydrous application is that when NH3 is placed in the soil it immediately turns into NH4 by striping H from H2O. This action releases OH into the soil in increases pH, depending on rate pH can reach 10.0 this hike in soil pH is a short term as the system disperrses and NH4 immediately begins the conversion to NO3 release H and driving down pH. The high pH in itself is not the problem but if the pH is still high and soil dries the OH will strip H from NH4 and NH3 is formed. The ammonia gas (NH3) is what can easily damage the sensitive seedling.
After fielding several calls in one day I wanted to dig a bit deeper and see what the science and specialist say. I was hoping for a nice consensus, haven’t found that yet. Here are some snip-its.
From Kansas State University
Dr. Dave Mengel
As a general rule, wait about 7 to 10 days between the anhydrous ammonia application and wheat planting. The higher the nitrogen rates and the wider the spacing (creating a higher concentration of ammonia in the band), the longer period of time you should wait. Also, in dry soils you may need to wait longer.
Canada Grains Council’s Complete Guide to Wheat Management Link
In the past, it was recommended that seeding be delayed for two days after banding anhydrous ammonia (NH3). However, in many soils as long as the NH3 is placed 5- 7.5 cm ( 2-3 inches) away from the seed, NH3 can be applied at the time of seeding. Seed damage from NH3 is most likely to occur under dry conditions on sandy soils when there is insufficient separation from the seed. Placement of fertilizer nitrogen should be deeper in sandy soils than in loams or heavy textured soils. Narrow band spacing 25 to 30 cm (10-12 in) is better than wider band spacing particularly under low moisture conditions.
From University on Minnesota
Peer reviewed publication
VARVEL: EFFECTS OF ANHYDROUS AMMONIA ON WHEAT AND BARLEY AGRONOMY JOURNAL, VOL. 74. NOVEMBER-DECEMBER 1982
Field experiments were conducted 1979-1981 on a Wheatville loam soil. The treatments consisted of three rates of N as anhydrous ammonia (45, 90, and 135 kg/ha) in 1979 and four rates of N (0, 45, 90, and 135 kg/ ha) in 1980-1981 at three depths (8,16, and 24 cm) in all combinations. Spring wheat and barley were then seeded at three different times. Seedling stand counts, grain yield, and protein were used to determine the effect of the treatments. Seedling stands were reduced in some cases, but no reduction in grain yield or protein was obtained due to the reduction in stand. The most important factor in spring anhydrous application was the depth of application, which caused greater moisture loss and seedbed disruption at the 24-cm application depth.
Spring wheat and barley response to N rates was similar at all depths of application (no significant interaction between N rate and application depth). The results indicate that anhydrous ammonia can be applied safely at planting time on spring wheat and barley, if applied at the 8 to 16 cm depth and at N rates currently used in the northern Great Plains.
From University on Minnesota (referring to corn) link
The only risk of planting soon after AA application is if seeds fall within the ammonia retention zone. To avoid seedling injury separation in time or space can be important. Under ideal soil moisture conditions and proper application depth of a typical agronomic rate normally there is little risk of seedling injury even if planted on top of the application zone right after AA application. That said, this can be risky and I would not recommend planting on top of the AA row. If you have RTK guidance it is very easy to apply AA between the future corn rows. If RTK guidance is not an option, I would recommend applying AA on an angle to the direction of planting to minimize the potential for planting on top of the AA band. If application conditions are less than ideal and you have no RTK guidance to ensure a safe distance from the AA band, then waiting 3 to 5 days before planting is typically enough time to reduce the risk of seedling injury.
From University on Wisconsin (referring to corn) Link
The depth of NH3 placement was the greatest factor in determined potential seedling damage. The time after application had little impact.
Iowa State University (referring to corn)
by Regis Voss, extension agronomist, Department of Agronomy
The wet fall and spring will cause anhydrous ammonia application and corn planting date to be close. This will lead to the oft asked question, “How long do I have to wait to plant corn after ammonia application?” If there is a soil separation between the ammonia zone and the seed, planting can be done the same day the ammonia is applied. If the seed is to be placed in the ammonia zone, the longer the waiting period the less potential for root injury. There is no magic number of days to wait.
My take home from several hours of reading research articles and factsheets was my favorite answer IT DEPENDS. I believe Regis Voss with ISU had it right, there is no magic number. The important aspects for determining time will be 1) Soil Moisture 2) N rate 3) Depth and 4) shank spacing. From the reading I think there may be some general rules of thumb.
On the conservative side with good soil moisture, NH3 placed at 6″ deep, rate below 80 lbs and spacing of about 15″ the next day should be ok. As any one of these factors change (drier soil, higher rates, shallower application, wider rows) the more time should be added to reduce risk. One thing to consider is field variability. While the field on average may have great moisture there could be dry spots, while on average you are 6″ deep with the NH3 there are areas the rig is bound to rise up and go shallow. So there is always a chance for hot spots. All of that said I could not find any research on this topic for winter wheat in the southern Great Plains much less Oklahoma. I will always tend to the safe side and suggest if possible to delay sowing a few days after applying anhydrous. However if time is critical proceed with caution.
Looks like I can add one more project to my list and I need to find some open ground and do some “Experimenting”.
Happy Sowing All!
NDVI, Its not all the same.
With the most recent FAA UAV announcement my phone has been ringing with excited potential UAV users. Two points always comes up in the conversation. NDVI (normalized difference vegetation index) and image resolution. This blog will address the use of NDVI, resolution will come later. Before getting into the discussion, what NDVI is should be addressed. As described by Wikipedia, NDVI is a simple graphical indicator that can be used to analyze remote sensing measurements, typically but not necessarily from a space platform, and access whether the target being observed contains live green vegetation or not. NDVI is a mathematical function of the reflectance values of two wavelengths regions, near-infrared (NIR) and visable (commonly red).

Calculation for NDVI. Any visible wavelegnth can be substituted for the red wavelength.
The index NDVI has been tied to a great number of crop factors, the most important being biomass. Biomass being important as most things in the plant world impact biomass and biomass is related to yield. The most challenging issue with NDVI is it is highly correlated with biomass and a plants biomass is impacted by EVERYTHING!!!! Think about it, how many things can impact how a plant grows in a field.

Image showing the impact of nitrogen on a potted plants spectral reflectance pattern. The yellow line has 0 Nitrogen and the orange line had 100 lbs. The higher the line the more that wavelength is reflected. Note Photosynthetic wavelength are absorbed more (reflected less) when the plant is bigger but the NIR (right side) is absorbed less by the healthier plants.
The kicker that most do not know is that all NDVI’s values are not created equal. The source of the reflectance makes a big difference.
Measuring reflectance requires a light source, this is where the two forms of NDVI separate. Passive sensors measure reflectance using the sun (natural light) as a light source while active sensors measure the reflectance from a known light source (artificial light). The GreenSeeker is a good example of a active sensor, it emits its own light using LEDs in the sensor while satellite imagery is the classic passive sensor.

Picture representation of satellite remote sensing. http://www.crisp.nus.edu.sg/~research/tutorial/optical.htm
The challenge with passive remote sensing lies within the source of the light. Solar radiation and the amount of reflectance is impacted by atmospheric condition and sun angle to name a few things. That means without constant calibration, typically achieved through white plate measurements, the values are not consistent over time and space. This is the case whether the sensor is on a satellite or held held. In my research plots where I am collecting passive sensor data, so that I can measure all wavelength, I have found it necessary to collected a white plate calibration reading every 10 to 15 minutes of sensing. This is the only way I can remove the impacts of sun angle and cloud cover. When using the active sensors as long as the crop does not change the value is calibrated and repeatable.
What does this mean for those wanting to use NDVI collected from a passive sensor (satellite, plane, or UAV)? Not much if the user wants to distinguish or identify high biomass and low biomass areas. Passive NDVI is a great relative measurement for good and bad. However many who look at the measurements over time notice the values can change significantly from one day to the next. The best example I have for passive NDVI is a yield map with no legend. Even the magnitude of change between high and low is difficult to determine.
Passive NDVI in the hands of an agronomist or crop scout can be a great tool to identify zones of productivity. It becomes more complicated when decisions are made solely upon these values. One issue is this is a measure of plant biomass. It does nothing to tell us why the biomass production is different from one area to the next. That is why even with an active sensor OSU utilizes N-Rich Strips (N-Rich Strip Blog). The N-Rich Strip tells us if the difference is due to nitrogen or some other variable. We are also looking into utilizing P, K, and lime strips throughout fields. Again a good agronomist can utilize the passive NDVI data by directing sampling of the high and low biomass areas to identify the underling issues creating the differences.
OkState has been approached by many UAV companies to incorporate our nitrogen rate recommendation into their systems. This is an even greater challenge. Our sensor based nitrogen rate calculator (SBNRC blog) utilizes NDVI to predict yield based upon a model built over that last 20 years. That means to correctly work the NDVI must be calibrated and accurate to a minimum of 0.05 level (NDVI runs from 0.0 to 1.0). To date none have been able to provide a mechanism in which the NDVI could be calibrated well enough.
Take Home
NDVI values collected with a passive sensor, regardless of the platform the sensor is on, has agronomic value. However its value is limited if the user is trying to make recommendations. As with any technology, to use NDVI you should have a goal in mind. It may be to identify zones or to make recommendations. Know the limitations of the technology, they all have limitations, and use the information accordingly.
2015-16 Wheat Crop Nitrogen Review
From trials to phone calls (and text messages, and tweets, and ect. ect) I have gathered a fairly good picture of this years winter wheat nitrogen story. And as normal, nothing was normal. Overall I seen/heard three distinct trends 1) Did not take much to make a lot 2) took a ton to make a lot 3) saw a response (N-rich strip or cow-pow) but fertilizer never kicked in. Covers most of the options, doesn’t it.

The N-rich strips really came out over all very good this year. N-Rich Strip Blog. On average many of those using the N-Rich Strip and SBNRC (SBNRC Blog) producers have been getting in the neighborhood of 1.0-1.3 lbs of N applied per bushel produced. This year the numbers ran from 0.66 to 2.3 lbs of N per bushel. In both extremes I believe it can be explained via the field history and the N-Cycle.

Nitrogen Cycle Pete’s Sheet
In at least two fields, documented with calibrated yield monitors, the N-Rich Strip and SBNRC lead to massive yields on limited N. One quarter of IBA bumped 86 bpa average on 47 lbs of N while a second quarter, also IBA, managed 94 bpa average on about 52 units of N. We are currently running grain samples from these fields to look protein levels.
The other side of the boat were those with N-Rich strip calling for +2.0 lbs N per bushel. I had received notes from producers without N-rich strips saying that they could predict yield based on the amount of N applied and it was a 2 to 1 ratio. Not always but many of these high N demand fields where wheat following a summer or double crop or corn or sorghum. While many of the low N demand fields were wheat after wheat or wheat after canola. In a rotational study that had been first implemented in the 2014-15 crop year I saw big differences due to previous crop. The picture below was taken in early March. The straw residue in wheat after wheat had just sucked up the nitrogen. While it was evident the residue from the canola broke down at a much more rapid pace releasing any and all residual nutrients early.
The yield differences were striking. The canola rotation benefited the un-fertilized plots by 22 bpa and even with 90 lbs of N applied having canola in the rotation increased yields by 12 bpa. We are looking and grain quality and residual soil sample now. I am sure there will be a more indepth blog to follow.
Another BIG story from the 2015-16 wheat crop was the lack of benefit from any N applied pre-plant. It really took top-dress N this year to make a crop. Due to our wet early fall and prolong cold winter N applied pre was either lost or tied up late. Work by Dr. Ruans Soil Fertility Program really documented the lack luster pre-plant N effect. The figure below shows 4 location of a rate by timing student. The number at the bottom of each graph is a rate by time (30/0 means 30 lbs Pre-0 lbs Top, 60/30 means 60 lbs Pre-30 lbs Top). At every single location 0/60 beat 60/0. Top-dress N was better than Pre-plant N.

Figure 1. Work from Ethan Driver and Dr. Bill Raun. Study looked at rate and timing of N fertilization in wheat. Treatments are ordered by total N applied.
The last observation was lack of response from applied N even though the crop was deficient. Seen this in both the NE and NW corners. I would hazard with most of the circumstance it was due to a tie up of applied N by the previous crops residue. The length at which the winter stretched into spring residue break down was also delayed.
Take Home
Here it is folks APPLY NITROGEN RICH STRIPS. Just do it, 18 years of research preformed in Oklahoma on winter wheat says it works. Hold off on heavy pre-plant N even if anhydrous is cheap. It does matter how cheap it is if it doesn’t make it to the crop. Will we see another year like 2015-16, do not know and not willing to place money on either side. What we do know is in Oklahoma split applying nitrogen allows you to take weather into account and the N-Rich strip pays dividends.
There are several fact sheets available on top-dressing N and the application of N-Rich strips. Contact your local Oklahoma Cooperative Extension Service county educator to get a copy and see if they have a GreenSeeker sensor on hand.
DAP vs MAP, Source may matter!
Historically the two primary sources of phosphorus have had different homes in Oklahoma. In general terms MAP (11-52-0) sales was focused in Panhandle and south west, while DAP (18-46-0) dominated the central plains. Now I see the availability of MAP is increasing in central Oklahoma. For many this is great, with MAP more P can be applied with less material. which can over all reduce the cost per acre. There is a significant amount of good research that documents that source of phosphorus seldom matters. However this said, there is a fairly large subset of the area that needs to watch what they buy and where they apply it.
If you are operating under optimum soil conditions the research shows time and time again source does not matter especially for a starter. In a recent study just completed by OSU multiple sources (dry, liquid, ortho, poly ect ect) of P were evaluated. Regardless of source there was no significant difference in yield. With the exception of the low pH site. The reason DAP was so predominate in central Ok, soil acidity. See an older blog on Banding P in acidic soils.

Figure 1. The cover of an extension brochure distributed in Oklahoma during the 1980s.
When DAP is applied, the soil solution pH surrounding the granule will be alkaline with a pH of 7.8-8.2. This is a two fold win on soil acidity aka aluminum (Al) toxicity. The increase in pH around the prill reduces Al content and extends the life of P, and as the pH comes back down the P ties up Al and allows the plant to keep going. However, the initial pH around the MAP granule ranges from an acid pH of 3.5-4.2. There is short term pH change in the opposite direction of DAP, however the the Al right around the prill becomes more available and in theory ties up P even faster.
Below is a table showing the yield, relative to untreated check, of in-furrow DAP and MAP treatments in winter wheat. The N401 location had a ph 6.1 while Perk (green) has a pH of 4.8. At Perkins in the low pH, both forms of P significantly increased yeild, almost 20 bushel on the average. DAP however was 5 bushel per acre better than MAP. At the N40 site the yield difference between the two sources was 1 bushel.
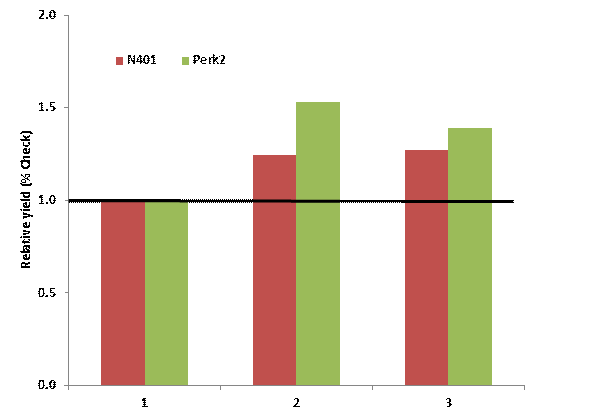
Relative yield winter wheat grain yield MAP and DAP both applied at equal rates of P (32 lbs P2O5 ac) when compared to a untreated check.
In general it can be said that in acid soils DAP will out preform MAP while in calcareous high pH soils MAP can out preform DAP. So regarding the earlier statement about the traditional sales area of MAP or DAP if you look at the soil pH of samples went into the Oklahoma State University Soil, Water, and Forage Analytical lab the distribution makes since.

Average soil pH of samples sent into OSU soil water forage analytical lab by county.
In the end game price point and accessibility drives the system. In soils with adequate soil pH levels, from about 5.7 to around 7.0, get the source which is cheapest per lbs of nutrient delivered and easiest to work with. But if you are banding phosphorus in row with your wheat crop because you have soil acidity, DAP should be your primary source.
Herbicide and UAN tank mixed for top-dress
Spring is the time that many wheat producers apply herbicide and nitrogen (N) fertilizer. For many this can be accomplished in a single pass by tank mixing the herbicide and UAN. In most cases this is an effective practice which eliminates one pass over the field. There are some scenarios in which this practice is ill advised. One such scenario is high temperatures which would lead to excessive leaf burn and crop damage. The other scenario is no-till and that will be the focus of this article. Ruling out warm temperature tank mixing herbicides and nitrogen, assuming the herbicide can be tank mixed, is a good practice. No-till on the other hand can be a different issue.

Situations with a lot of residue and smaller wheat is common during top-dress.
The problem in no-till comes from the liquid application method needed to apply herbicides, flat flan. To get a good kill with the herbicide the spray pattern needs to have good coverage, i.e a lot of small droplets to ensure maximum surface area impacted. Unfortunately there are four primary fates of UAN when applied via flat fan nozzles. The UAN could be taken directly up into the wheat plant via absorption through the leaves, the UAN could reach the soil and go into the soil solution or absorbed onto the soil itself, the UAN can be taken up by weeds, or the UAN droplet may hit dead plant tissue and be adsorbed into the residue.

UAN applied with a flat fan will hit a growing plant, the soil, or residue.
The fourth fate of UAN presented is what can make the tank mix less efficient than a two pass system. In a no-till system any UAN that hits residue should be counted as lost, for the short term. The decision to go with a one pass or two pass system can be aided by evaluating the amount of canopy coverage. For example if the no-till field has 50% canopy coverage then one could estimate 50% of the UAN applied via a one pass system would be tied up in the residue. The cost of a second application could then be compared to the lost N. If 15 gallon of 28-0-0 was being applied then approximately 22.5 lbs of N would be tied up by the straw. At a price of $0.40 per lb on N, that is $9.00 worth of N. Conversely if the canopy coverage was 80% only 20% or 9 lbs of N would be tied up in the residue. Saving the $3.60 in nitrogen would not justify a second trip over the field. Luckily OSU recently released the Canopeo app which uses a cell phones camera to take pictures and quickly and accurately determine % canopy coverage. Canopeo is available for iOS and android http://canopeoapp.com/.
In fields with a high amount of residue or limited canopy coverage UAN should be applied with streamer nozzles. This will concentration the fertilizer into streams which will allow the UAN to have enough volume to move off the residue and into the soil.
So as the decision is being made to tank mix herbicide and UAN or make two passes take into consideration: % canopy coverage, rate of UAN (how much could be lost), cost of UAN per pound, and cost of a second trip over the field.
Below is an excerpt from the publication Best Management Practices for Nitrogen Fertilizer in Missouri; Peter C. Scharf and John A. Lory. http://plantsci.missouri.edu/nutrientmanagement/nitrogen/practices.htm
Broadcasting UAN solution (28 percent to 32 percent N) is not recommended when residue levels are high because of the potential for the N in the droplets to become tied up on the residue. Dribbling the solution in a surface band will reduce tie-up on residue, and knife or coulter injection will eliminate it. Limited research suggests that the same conclusions probably apply for grass hay or pasture. Broadcast UAN solution is also susceptible to volatile loss of N to the air in the same way as urea, but only half as much will be lost (half of the N in UAN solution is in the urea form).
Canola fertilizer rates when skip row seeding.
When drilling canola a common strategy to improve seeding rate accuracy is to only use every other row which effectively doubles the rate of seed going through each meter. There are also many producers who utilize air seeders and just prefer the wider spacing. Every season I get several questions about determining total fertilizer rates if the seed is dropped every other row but fertilizer is dropped every row. Regardless of whether or not fertilizer goes down every row it is important that the amount of salts placed with seed does not exceed the limit. The table below provide the limits in terms of lbs of salt per acre. If using 18-46-0 (DAP) or 11-52-0 (MAP) this is equivalent to pounds of N per acre. However if the fertilizer you use contains potassium (K) or sulfur (S), those have to be considered. An easy rule of thumb for determining total salt level of a fertilizer is pounds of N + K + 1/2 S.

Maximum amount of salt that can be applied in furrow with canola seed. Application rate should be at or below this value.
In a scenario in which canola is seeded in skip rows but every row will get fertilizer the total amount of fertilizer can be doubled. For example on a 15″ row spacing the max salt rate is 5 lbs per acre. If you were using DAP as your starter that maximum rate to place in furrow would be 28 lbs of DAP per acre. If using a drill set of 7.5″ spacing and putting fertilizer down every row the max rate would increase up to 56 lbs DAP per acre.

Seed, colored blue, is placed in every other row while an equal amount of fertilizer, dark grey circles, is placed in every row.
Some producers may have the capability of applying different rate in every other row. In this scenario it is important to maintain that safe rate in the seed furrow. In the opposite row, fertilizer rate can go as high as you wish or the equipment can handle.

Seed, colored blue, is placed in every other row while fertilizer, dark grey circles, is placed in every row. In this scenario a high rate is placed in the row without seed.
Now the big question is, “Is between row fertilization a good idea?” While we do not have results on this style of application (trials will be going out this year) we can draw upon upon similar work in other crops. For me the best win would be the second scenario in which a higher rate could be place between the rows. In this row I would use a urea and DAP blend. Any time we can put urea below the soil surface its a win and in fields with very soil soil test phosphorus (P) it would create something similar to the deep P bands once popular in corn production. Now if the field had adequate soil test P, I would focus on urea between rows. Keep in mind it is never a good to place urea in furrow with canola seed. For the average producer who is using a box drill the first scenario is the only option. In this case the rate of the between row bands will be reduced however I still believe on fields with very low soil test P this is potentially a great way to get the rest of it on. Remember if on 15″ and using DAP max rate only gets 12.9 lbs of P2O5 down. If fertilizer is dropped down every tube that number increases to about 26 lbs P2O5, which is still not enough for fields with low soil test P, but is better. With hope we will have some good results to share from the 2015-2016 canola crop.
Some thoughts on pre-plant nitrogen and a little outside the box thinking
It is that time of year, every Co-op I drove by the other day had a line of trucks pulling anhydrous tanks and the spinner spreaders were being loaded. For those of you who haven’t applied your nitrogen yet lets discuss the options traditional and nontraditional.
Anhydrous Ammonia, 82-0-0: by far the most widely used N source is the southern Great Plains. While it is not the most enjoyable to work with it is the cheapest per pound of N and that leads to its wide spread use without Oklahoma wheat production. Just a few simple rules with NH3, get it in the ground and close the row behind you. In conventional till this is usually easier unless the ground is too wet or too dry. In no-till this may be a little more challenging but usually easily accomplished. With the rise in low disturbance applicators I am seeing more and more acres of no-till receiving NH3. Last year I was in a field of stripper stubble and I had a hard time finding where the rig had run, minus wheel tracks.
Urea, 46-0-0: is second on the hit list in nitrogen sales in our state. It is a safe source that is easily handled and applied. In a conventional till system where the urea can be worked in shortly after application it is a very efficient and effective source. Unfortunately when it is applied to the soil surface and rain is the method of incorporation we can experience between 5-60% N losses. The losses come from how urea is converted to plant available ammonium (NH4). For urea (NH2)2CO2, to be converted to plant available NH4 it needs the enzyme urease. Urease is present everywhere but in the highest concentrations on plant residue. The figure below shows the reaction, urease converts urea into NH3 as soon as the prill dissolves. In the presence of moisture the NH3 (gas) is turned immediately to NH4 (solid) and is absorbed onto the soil particle.
The problems come when there is no soil particle for the NH4 to bind with. It usually takes 0.50 inches of rain or irrigation to fully dissolve and incorporate urea into the soil. So if we only get a few tenths or hundredths, even heavy dews, some of the urea will dissolve, be converted to NH3 then NH4 and be left on the plant/residue. When the moisture dries, some or all of the NH4 goes back to NH3 and will gas off into the atmosphere. I have even seen this happen when urea is applied on a wet/damp soil, not incorporated and it doesn’t rain for significant period of time. If the temps are cooler the urease is slower so less of the urea is converted to NH4, but if the temps are warm 60+ degrees these little enzymes can act very quickly.

Urea placed on the surface of a wet soil under two temperature regimes. White text is the number of hours after application.

Urea placed on dry soil, Top row: dry soil no water added, Bottom left, moisture added from subsurface, Bottom right : simulated rain fall event of 1/2″. White text is the number of hours after application.
Below is a short video on using urea fertilizer.
While the recent rains are a blessing and will surely help germination, it is not aiding our N use efficiency especially in no-till. That is why in some parts of the state you may see some grain drills running right now. Some of those producers are not planting wheat they are actually applying there pre-plant urea. I have even been told in the SW part of the start some producers are using air-seeders to apply their urea. While this seems like a costly venture I have worked with the Ag Economist to create a calculator to figure up the break even for when it would pay to use an air-seeder over the traditional spinner spreader in no-till. We hope to put the finishing touches on it in the next few days. When it is completed it will be shared on this blog.
Liquid Urea Ammonium Nitrate, 32-0-0 or 28-0-0: while this is one of the more expensive forms of N many producers are utilizing this source because the can pre buy and store on site and as sprayer get larger they can cover a significant amount of ground quickly. For the most part UAN is used in no-till and is a great source. I always recommend that applicators use streamer nozzle or streamer bars to apply UAN. When UAN is applied via a flat fan nozzle it spreads the fertilizer across the residue allowing a significant portion to be tied up. The streamers concentrate the fertilizer into streams/bands reducing contact with residue and increasing the amount of UAN that reaches the soil surface.
Timing and Rates
The cost of anhydrous, about $0.1 to 0.12 less per pound N less than urea is driving its use this year. The lower price is also driving a significant about of producers to go with 100% of their N pre-plant. While this makes for sound economics now having all of your N upfront is like putting all of your eggs in one basket. If we do get that cold and wet winter as some are calling for this presents a great chance for the N to move down the soil profile and down the slope. I have always recommended split application. This allows a producer to judge the crop throughout fall, winter and even yearly spring and adjust his or her N plan accordingly. For those who plan to graze there is still a need to get enough N down to produce fall forage, this may be 50 to 80 lbs of N, but for grain only production planted later in the fall a typical crop may only need 20-30 lbs of N before going into winter. The old rules of thumb, 2 lbs N per bushel and 30 lbs N per 100 lbs of gain still work and are better than a guestimate but we have better ways. Right now is the time to plan to apply N-rich strip, a strip in the field with 40 to 50 lbs more than the rest of the field. These strips can be applied with a variety of applicators, but as long as the N goes down in at least an area 10 ft wide by 300 ft long it is good to go.

Just a few of the applicators used for putting out N-Rich Strips. ATV Sprayer, Receiver Hitch mounted Sprayer, Road sprayer with a rear boom, pull type spinner, large sprayer, push spreader. Not shown is NH3 applicator.
Below is a N-Rich Strip 101 video.
If you have got the N-Rich strips out you can set back and watch to see when and if they develop. If you can see the strip you know you need too fertilize.
While many are not ready to think about top-dressing yet, it is never too early. Don’t be afraid to think outside the box. Oklahoma’s springs tend to present the perfect conditions for N loss when urea is the primary N source. This year in a 4R Top-dress Nitrogen Application Demo, at Lahoma and Chickasha, we are going to apply just about every available commercial source in about every possible manor. Urea will be broadcast, coated with inhibitors, applied with a grain drill, NH3 will be knifed in, and UAN will be applied with flat fan nozzles, streamer nozzles and knifed in. As technologies improve and the cost of N remains relatively high the options for top-dress N application will continue to improve. The economics of wheat production don’t look great right now so don’t be afraid to think outside the box, even if it does raise the eyebrows of your neighbors. Fill free to contact myself or your local extension educator if you have any questions about N application.
Sampling for pH and liming in continuous no-till fields
This article is written by Dr. David Mengel, Kansas State University Soil Fertility Specialist.
One question that commonly comes up with continuous no-till operations is: “How deep should I sample soils for pH?” The next common question is: “How should the lime be applied if the soil is acidic and the field needs lime?”
Sampling depth in continuous no-till
First, sampling depth. Should two sets of samples be taken, at different depths?
Our standard recommendation for pH is to take one set of samples to a 6 inch depth. On continuous no-till fields where most or all of the nitrogen (N) is surface applied, we recommend taking a second sample to a 3-inch depth. We make the same recommendation for long-term pasture or grass hayfields, such as a bromegrass field that has been fertilized with urea annually for several years.
Nitrogen fertilizer is the primary driving force in lowering soil pH levels, so N application rates and methods must be considered when determining how deep to sample for pH. In no-till, the effects of N fertilizer on lowering pH are most pronounced in the area where the fertilizer is actually applied. In a tilled system, the applied N or acid produced through nitrification is mixed in through the action of tillage and distributed throughout the tilled area.
Where N sources such as urea or liquid UAN solutions are broadcast on the surface in no-till system, the pH effects of the acid formed by nitrification of the ammonium will be confined to the surface few inches of soil. Initially this may be just the top 1 to 2 inches but over time, and as N rates increase, the effect of acidity become more pronounced, and the pH drops at deeper depths. How deep and how quickly the acidity develops over time is primarily a function of N rate and soil CEC, or buffering capacity.
Where anhydrous ammonia is applied, or liquid UAN is knifed or coulter banded below the surface, an acid zone will develop deeper in the soil, usually 2-3 inches above the release point where the fertilizer is placed in the soil. So if the ammonia is injected 8 inches deep, there will be acid bands 5 to 8 inches below the soil surface. As with long-term surface applications, these bands will expand over time as more and more N fertilizer is placed in the same general area. The graphic below illustrates the effect of a high rate of ammonia placed in the same general area in the row middle on a high CEC soil for more than 20 years.
The actual depth of the acid zone in fields fertilized with ammonia gets tricky as application depth can vary depending on the tool used to apply the ammonia. Traditional shank applicators generally run 6 to 8 inches deep, so a sample for pH measurement could be taken at 3-6 inches or 5-8 inches deep, depending on how deep the shanks were run. The new low-disturbance applicators apply the ammonia 4-5 inches deep. A sweep plow or V-blade applies ammonia only 3-4 inches deep. So sampling depth for pH should really depend on where the acid-forming N fertilizer is put in the soil.
Liming application methods in continuous no-till
Now, where do you place the lime in continuous no-till? If you surface apply N, then surface apply the lime. That’s a simple but effective rule. But remember that surface-applied lime will likely only neutralize the acidity in the top 2-3 inches of soil. So if a producer hasn’t limed for 20 years of continuous no-till and has applied 100 to 150 pounds of N per year, there will probably be a 4-5 inch thick acid zone, and the bottom half of that zone may not be neutralized from surface-applied lime. So, if a producer is only able to neutralize the top 3 inches of a 5-inch deep surface zone of acid soil, would that suggest he needs to incorporate lime? Not really. Research has shown as long as the surface is in an appropriate range and the remainder of the acid soil is above pH 5, crops will do fine.
Liming benefits crop production in large part by reducing toxic aluminum, supplying calcium and magnesium, and enhancing the activity of some herbicides. Aluminum toxicity doesn’t occur until the soil pH is normally below 4.8. At that pH the Al in soil solution begins to increase dramatically as pH declines further. Aluminum is toxic to plant roots, and at worse the roots would not grow well in the remaining acid zone.
This implies that the acid zones from ammonia are probably not a major problem. We have monitored ammonia bands in the row middles of long-term no-till for many years and while the pH got very low, below 4.5, we never saw any adverse impacts on the crop that would justify liming and using tillage to incorporate the lime. In fact, some nutrients such as zinc, manganese, and iron can become more available at low pH, which can be an advantage at times.
Yield enhancement is not the only concern with low-pH soils, however. Herbicide effectiveness must also be considered. The most commonly used soil-applied herbicide impacted by pH is atrazine. As pH goes down, activity and hence performance goes down. So in acid soils weed control may be impacted. We do see that in corn and sorghum production.
Liming products for no-till
When choosing a liming product, is there any value to using dolomitic lime (which contains a large percentage of magnesium in addition to calcium) over a purely calcium-based lime product? On most of our soils in Kansas we are blessed with high magnesium content. So as long as we maintain a reasonable soil pH, there normally is enough magnesium present to supply the needs of a crop. Calcium content is normally significantly higher than magnesium, so calcium deficiency is very, very rare in Kansas. The soil pH would need to be below 4.5 before calcium deficiency would become an issue. Before calcium deficiency would occur, aluminum toxicity or manganese toxicity would be severely impacting crop growth. So producers really don’t have to worry about a deficiency of calcium or magnesium on most Kansas soils.
What about the use of pelletized lime as a pH management tool on no-till fields? The idea has been around for a while to use pel-lime in low doses to neutralize the acidity created from nitrogen and prevent acid zones from developing. There is no reason it won’t work, if you apply enough product each year. Pel-lime is a very high-quality product, normally having 1800 to 2000 pounds of effective calcium carbonate (ECC) per ton, and can be blended with fertilizers such as MAP or DAP or potash easily.
But it is costly. As an example, at a cost of $160 per ton and 1,800 lbs effective calcium carbonate (ECC) per ton, 100 pounds of ECC pel-lime costs $8.80. If it costs $25 per ton to buy, haul, and apply a 50% ECC limestone, that equates to $2.50 per 100 pounds ECC.
If you were applying 100 pounds of urea-based nitrogen, it would take approximately 180 pounds of ECC to neutralize the acidity produced by the N. This would require 200 pounds of 1,800 pound ECC pel-lime or 360 pounds of 50% ECC ag lime. The cost would be around $16 per acre with pel-lime or $4.50 per acre with ag lime. So technically, the pel-lime option is fine. But it would cost more than 3 times as much, at least in this example. You can use your own figures regarding costs and ECC of different lime products available to you to do a similar calculation. Deciding which product to use is a simple economic choice.
Summary
Applying N fertilizer to soil will cause the soil to become acidic over time. Placement of the applied N and the level of soil mixing done through tillage determine where the acid zones will develop. Make sure your soil testing program is focused on the area in the soil becoming acidic, and apply the lime accordingly.
Dave Mengel
Kansas State University
Professor Soil Fertility Specialist
dmengel@ksu.edu



















