Thoughts from an Agronomist- 1 Management of the Primordia
Josh Lofton, Cropping Systems Specialist
Many crop management recommendations emphasize actions that must be taken well before a crop reaches what we often call “critical growth stages.” Management this early can seem counterintuitive when the crop still looks small, healthy, or unchanged aboveground. However, much of a crop’s yield potential is determined early in the season at a level we cannot see in the field. Long before flowers, tassels, or heads (or any reproductive structure) appear, the plant is already making developmental decisions that shape its final yield potential. Understanding this “behind the scenes” process helps explain why timely, early-season management is often more effective than trying to correct problems later.
At the center of this process is the shoot apical meristem, commonly referred to as the growing point. This tissue produces leaf and reproductive primordia, which are the earliest developmental stages of future everything in the plant. These primordia form well before the corresponding plant parts are visible. Once these structures initiate—or if they fail to begin due to stress—the outcome is permanent. The plant cannot later in the season go back and recreate leaf number, leaf size, or reproductive capacity. As a result, early environmental conditions and management decisions play a disproportionate role in determining yield potential.
Corn is a good example of how early development influences final yield. By the time corn reaches the V4 growth stage, the plant only has four visible leaves with collars, yet internally it is far more advanced. Most of the total leaf primordia that will eventually form the full canopy have already begun, and the potential size of the ear is starting to be established. During this stage, the growing point is still below the soil surface and somewhat protected from some stressors but highly susceptible to others. Nitrogen deficiency, cold temperatures, moisture stress, compaction, or herbicide injury at or before V4 can reduce leaf number and limit leaf expansion. Even if growing conditions improve later, the plant cannot replace leaf primordia that were never formed, which reduces its ability to intercept sunlight and support high yields.

As corn approaches tasseling (VT), the crop enters a stage that is visually and physiologically important. Pollination, fertilization, and early kernel development occur at this time, and stress can have a critical impact on kernel set. However, by VT, the plant has already completed leaf formation, and much of the ear size potential has already been determined several growth stages earlier. Management at VT is therefore focused on protecting yield rather than creating it. Late-season nutrient applications may improve plant appearance or maintain green leaf area, but they cannot increase leaf number or rebuild ear potential lost due to early-season stress. This distinction helps explain why some late inputs show limited yield response even when the crop looks responsive.
Grain sorghum provides another clear example of why early management is emphasized. Although sorghum often grows slowly early in the season and may appear unimportant during the first few weeks after emergence, the first 30 days are among the most critical periods in its development. During this time, the growing point is actively producing leaf primordia and transitioning from vegetative growth toward reproductive development. Head size potential is primarily established during this early window, and the plant’s capacity to support tillers is influenced by early nutrient availability and moisture conditions. Stress from nitrogen deficiency, drought, weed competition, or restricted rooting during the first 30 days can reduce head size and kernel number long before visible symptoms appear.
Once sorghum reaches later vegetative and reproductive stages, much like corn at VT, management shifts from building yield potential to protecting what has already been determined. Improving conditions later in the season can help maintain plant health and grain fill, but it cannot fully compensate for early limitations imposed at the primordial level. This is why early fertility placement, timely weed control, and moisture conservation are consistently emphasized in sorghum production systems.
Across crops, a typical pattern emerges: the growth stages we observe in the field often reflect decisions the plant made weeks earlier. When agronomists stress early-season management, they are responding to plant biology rather than simply following tradition. By the time visible “critical stages” arrive, the plant has already established many of the components that define yield potential.
The key takeaway is that effective crop management must be proactive rather than reactive. Early-season decisions support the crop while it is still determining how many leaves it can produce, how large its reproductive structures can become, and how much yield it can ultimately support. Waiting until stress becomes visible often means responding after the plant has already adjusted its potential downward. Recognizing what is happening at the primordial level helps explain why management ahead of critical stages consistently delivers the greatest return, even when the crop appears small and unaffected aboveground.
For questions or comments reach out to Dr. Josh Lofton
josh.lofton@okstate.edu
One Well-Timed Shot: Rethinking Split Nitrogen Applications in Wheat production
Brian Arnall, Precision Nutrient Management Specialist
Samson Abiola, PNM Ph.D. Student.
Nitrogen is the most yield limiting nutrient in wheat production, but it’s also the most unpredictable. Apply it too early, and you risk losing it to leaching or volatilization before your crop can use it. Apply it too late, and your wheat has already determined its yield potential; you’re just feeding protein at that point. For decades, the conventional wisdom has been to split nitrogen applications: put some down early to get the crop going, then come back later to apply again. But does splitting actually work? And more importantly, when is the optimal window to apply nitrogen if you want to maximize both yield and protein quality? We spent three years across different Oklahoma locations testing every timing scenario to answer these questions.
How We Tested Every Nitrogen Timing Scenario in Oklahoma Wheat
Between 2018 to 2021, we conducted field trials at three Oklahoma locations, including Perkins, Lake Carl Blackwell, and Chickasha, representing different soil types and growing conditions across the state. We tested three nitrogen rates: 0, 90, and 180 lbs N/ac, applied as urea at five critical growth stages based on growing degree days (GDD). These timings were 0 GDD (preplant, before green-up), 30 GDD (early tillering), 60 GDD (active tillering), 90 GDD (late tillering, approximately Feekes 5-6), and 120 GDD (stem elongation, approaching jointing). We also compared single applications at each timing against split applications, where half the nitrogen (45 lbs N ac-1) went down preplant, and the other half was applied in-season (45 lbs N ac-1).
The Sweet Spot: Yield and Protein at the 90 lbs N/ac Rate
Across all site-years, at the 90 lbs N/ac rate, timing had a significant impact on both yield and protein. The highest yields came from the 30 and 90 GDD timings, producing 62 to 66 bu/ac, with 60 GDD reaching the peak (Figure 1). Protein at these early timings stayed relatively modest at 13%. The 90 GDD timing delivered 62 bu/ac with 14% protein matching the yield of the 30 GDD application but pushing protein a percentage higher (Figure 2). The real problem appeared at 120 GDD. Delaying application until stem elongation dropped yields to just 49 bu/ac, even though protein climbed to 15%. That’s a 13 bushel penalty compared to the 90 GDD timing. At current wheat prices per bushel, that late application may cost farmers over $100 per acre in lost revenue. By 120 GDD, the crop has already determined its yield potential tillers are set, head numbers are locked in and nitrogen applied at this stage can only be directed toward protein synthesis, not building more yield components.
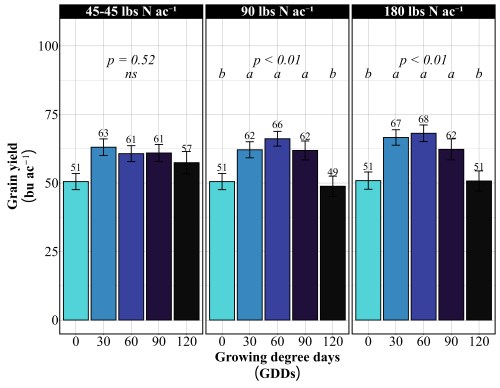
More Nitrogen Does not lead to high yield
Doubling the nitrogen rate to 180 lbs N/ac revealed something critical, more nitrogen doesn’t mean more yield. The yield pattern remained nearly identical to the 90 lbs N/ac rate. The 60 GDD timing produced the highest yield at 68 bu/ac, followed closely by 30 GDD at 67 bu/ac. The 90 GDD timing yielded 62 bu/ac, and the 120 GDD timing again crashed to 51 bu/ac. The only difference between the two rates was protein concentration (Figure 2). At 180 lbs N/ac, protein levels increased across all timings: 13% at preplant, 15% at both 30 and 60 GDD, 15-16% at 90 GDD, and 16% at 120 GDD. This confirms a fundamental principle: once farmers supply enough nitrogen to maximize yield potential, which occurred at 90 lbs N/ac in these trials, additional nitrogen only increases grain protein. It does not build more bushels. Unless farmers are receiving premium payments for high-protein wheat, that extra 90 lbs of nitrogen represents a cost with no yield return.
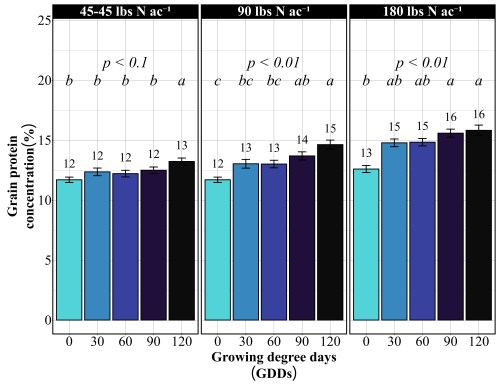
Should farmers split their nitrogen application?
Now that timing has been established as critical, the next question becomes: should farmers split their nitrogen applications, or is a single application sufficient? The conventional recommendation has been to split nitrogen apply part preplant to support early growth and tillering, then return with a second application later in the season to boost protein and finish the crop. But does the data support this practice? We compared three strategies at each timing: applying all nitrogen preplant, applying all nitrogen in-season at the target timing, or splitting nitrogen equally between preplant and in-season timing. The goal was to determine whether the extra trip across the field will deliver better results.
Our findings revealed that splitting provided no consistent advantage. At 30 GDD, all three strategies preplant, in-season, and split performed identically, producing 62-65 bu/ac with 12-13% protein (Figure 3 and 4). No statistical differences existed among them. At 60 GDD, similar pattern was held. Yields ranged from 61 to 66 bu/ac and protein stayed at 12-13% regardless of whether farmers applied all nitrogen preplant, all at 60 GDD, or split between the two. At 90 GDD, the single in-season application actually outperformed the split. While yields remained similar across all three methods (61-64 bu/ac), the in-season application delivered significantly higher protein at 13.7% compared to 12.4% for preplant and 12.5% for split applications. This suggests that concentrating nitrogen at 90 GDD, rather than diluting it across two applications, allows more efficient incorporation into grain protein. The only timing where splits appeared beneficial was 120 GDD, where the split application yielded 59 bu/ac compared to 51 bu/ac for the single late application. But this is not a win for splitting, it simply demonstrates that applying all nitrogen at 120 GDD is too late and putting half down earlier salvages some of the yield loss. Across all timings tested, splitting nitrogen into two applications offered no agronomic advantage over a single well-timed application, meaning farmers are making an extra pass for no gain in yield or protein.
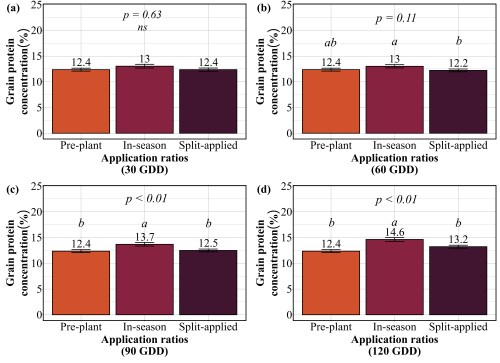
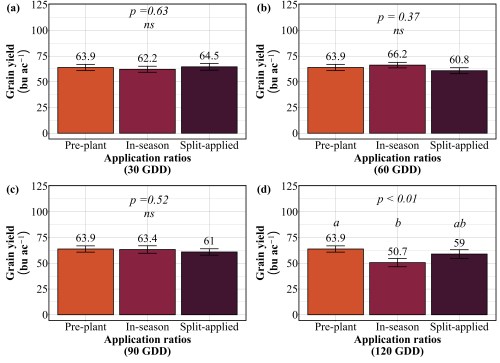
Practical Recommendations for Nitrogen Management
Based on three years of field data, farmers should target the 90 GDD timing (late tillering, Feekes 5-6) for their main nitrogen application to achieve the best balance between yield and protein. This window typically falls in late February to early March in Oklahoma, though farmers should monitor crop development rather than relying solely on the calendar apply when wheat shows multiple tillers, good green color, and vigorous growth. A rate of 90 lbs N/ac maximized yield in these trials; higher rates only increased protein without adding bushels, so farmers should only exceed this rate if receiving premium payments for high-protein wheat. Splitting nitrogen applications provided no advantage at any timing, meaning a single well-timed application at 90 GDD is sufficient for most Oklahoma wheat production systems. The exception would be sandy soils with high leaching potential, where splitting may reduce nitrogen loss. Farmers should avoid delaying applications until 120 GDD or later, as this timing consistently resulted in 15-25 bushel per acre yield losses even though protein increased. For farmers specifically targeting premium protein markets, a two-step strategy works best: apply 90 lbs N/ac at 90 GDD to establish yield potential and baseline protein, then follow with a foliar application of 20-30 lbs N/ac at flowering to push protein above 14% without sacrificing yield. Finally, weather conditions matter hot, dry forecasts increase volatilization risk and reduce uptake efficiency, so farmers should consider moving applications earlier if low humidity conditions are expected.
Split Application Caveat * Note from Arnall.
The caveat to the it only takes one pass, is high yielding >85+ bpa, environments. In these situation I still have not found any value for preplant nitrogen application. I have seen however a split spring application is valuable. Basically putting on 30-50 lbs at green-up, with the rest following at jointing (hollowstem). The method tends to reduce lodging in the high yielding environments.
This work was published in Front Plant Sci. 2025 Nov 6;16:1698494. doi: 10.3389/fpls.2025.1698494
Split nitrogen applications provide no benefit over a single well timed application in rainfed winter wheat
Another reason to N-Rich Strip.
Yet just one more data set showing the value of in-season nitrogen and why the N-Rich Strip concept works so well.
Questions or comments please feel free to reach out.
Brian Arnall b.arnall@okstate.edu
Acknowledgements:
Oklahoma Wheat Commission and Oklahoma Fertilizer Checkoff for Funding.
Using soil moisture trend values from moisture sensors for irrigation decisions
Sumit Sharma, Extension Specialist for High Plains Irrigation and Water Management
Kevin Wagner, Director, Oklahoma Water Resources Center
Sumon Datta, Irrigation Engineer, BAE.
Sensor based and data driven irrigation scheduling has gained interest in irrigated agriculture around the world, especially in semi-arid areas because of the easy availability of commercial irrigation scheduler technology such as soil moisture sensors and crop models. Moisture sensing has particularly gained interest among the agriculture community due to ease of availability of the sensors to the producers, affordable costs, and easy to use graphical user interface. Economic potential of sensors in saving irrigation costs, data interpretation training through extension education programs, and policy initiatives have also helped with adoption of the sensors, especially in the United States. However, sensor adoption and efficient use can still be challenging due to poor data interpretation, steep learning curves, overly high expectations and subscription costs. This blog briefly discusses scenarios where sensors can be helpful in irrigated agriculture. For moisture sensor types, functioning and installation, readers are referred to BAE-1543 OSU extension factsheet.

Irrigation Scheduling
Irrigation scheduling with soil moisture sensors follows traditional principles of field capacity (FC), plant available water, maximum allowable depletion (MAD), and permanent wilting point (PWP). Figure 1 shows the transition of soil moisture level from field capacity to MAD, and to permanent wilting point in a typical soil. The maximum amount of water that a soil can hold after draining the excess moisture is called field capacity. At this point, all the water in soil is available to the plants. As the moisture content in the soil declines, it becomes more difficult for the plants to extract moisture from the soil. The soil moisture level below which the available moisture in soil cannot meet the plant’s water requirement is called the MAD. The water stress that occurs once moisture level goes below this moisture level can cause yield reductions in crops. Therefore, irrigation should be triggered as soon as the soil moisture level approaches this point (MAD) to avoid any yield losses (for detailed information on MAD, its value for different soils and crops, and irrigation scheduling, readers are referred to BAE-1537). Modern soil moisture sensors can come self-calibrated and are equipped with water stress threshold levels for different crops to avoid water stress or overwatering (Figure 2). These decisions are useful in furrow and drip irrigation systems where irrigation triggers can be synchronized with MAD values.



Figure 2: Screenshots of graphic user interface of three sensors a) GroGuru b) Sentek c) Aquaspy (Top to bottom) with threshold levels for soil moisture conditions. Aquspy and Sentek credits: Sumit Sharma. GroGuru image credits: groguru.com
Soil Moisture Trends and Irrigation Depths
Soil moisture sensors can help make data-informed decisions about scheduling irrigation. Previous studies have shown that the moisture values may vary from one sensor to the other and may not represent the exact moisture levels in soil. However, all soil moisture sensors exhibit trends in recharge and decline in soil moisture conditions. These real time soil moisture trends can be used to make informed decisions to adjust irrigation and improve water use efficiency. In high ET demand environments of Oklahoma, pivots are usually not turned off during the peak growing season, yet sensors can help in making decisions for early as well as late growing periods.
One of the easiest adjustments that could be made using soil moisture sensor data is the adjustment of irrigation depth. In an ideal situation, every irrigation event should recharge the soil profile to field capacity; however, this is often limited by the crops’ water demand and the well/irrigation capacity to replenish soil moisture levels. Each peak in soil moisture detected by sensors shows irrigation or rain, which ideally should be bringing moisture to same level after irrigation. However, reduction in moisture peaks in the soil moisture profile with every irrigation often indicates greater crop water demand than what is replenished with irrigation. In such scenarios, as allowed by capacity and infiltration rates, the irrigation depth can be increased. These trend values are particularly useful for center pivot irrigation systems, where triggering irrigation based on MAD might lag due to time and space bound rotations of the pivots in Oklahoma weather conditions.

Figure 3: A screenshot from Aquaspy agspy moisture sensor showing moisture at 8” (blue) and 28” (red) with each irrigation event. Data and image credit: Sumit Sharma
Last irrigation can be a tricky decision to end the cropping season. For summer crops, this is the time when crop ET demand is declining due to decline in green biomass and cooler weather patterns. Similar moisture trends can be used to make decisions for the last irrigation events, which can be skipped or reduced if the profile moisture is good, or can be provided if profile moisture is low. This is important because in an ideal situation, one would want to end the season with a relatively drier profile to capture and store off-season rains. Additionally, saving water on last irrigation can save operational cost and potentially cover the cost of moisture sensor subscriptions.
These decisions can be illustrated with Figure 3, which shows the trends of declining and recharging in a soil profile under corn at 8- and 28-inch depth. This field was irrigated with a center pivot irrigation system which was putting 1-1.25 inches of water with each irrigation event; however, the peak water recharge rate at both depths was declining with each irrigation. This coincided with peak growth period indicating rising ET demand of the crop than what was replenished by the irrigation. Later, two rain events, in addition to irrigation, replenished soil moisture in both layers. As the pivot was already running at a slow speed, slowing it further was not an option without triggering runoff for this soil type and this well capacity. Further in the season, when the crop started to senesce and ET demand declined, each irrigation event added to the moisture level of the soil. This allowed the producer to shut down the pivot between 70% starch line and physiological maturity for the crop to sustain at a relatively wet soil profile and leave the soil in relatively drier profile for the off-season.
In high ET demanding conditions of Western Oklahoma, crops often rely on moisture stored in deep soil profiles during the peak ET period when well capacities can’t keep up with crop water demand. In the high ET demanding environments of Oklahoma, irrigated agriculture depends heavily on profile moisture storage. Declining soil profile moisture is common during peak ET periods in high water demanding crops such as corn. These observations are useful if one starts the season with considerable moisture in the soil profile, however such trends may be absent if the season is started with a dry soil profile. Dry soil profiles can be recharged early in the season with pre-irrigation or deeper early irrigations (if allowed by the infiltration rate of the soil), when crop ET demand is low, to build the soil moisture profile. As such, sensors can be used in reducing the irrigation depth or skipping irrigation in early cropping systems if one starts with a full profile. This usually allows root growth through the profile to chase the moisture in deeper layers. It should be noted that the roots will grow and chase moisture only if there is a wet profile, and not through a dry soil profile.
Sensor installation and calibration are important for efficient use of these devices in irrigation decision making. Poor installation can often lead to poor data and wrong decision making. Although modern sensors are self/factory calibrated, some do provide the option to adjust threshold levels manually based on field observations. Early installation of sensors can be useful in making informed decisions as soon as the season starts. For a more detailed analysis of proper sensor installation, refer to BAE-1543. Producers are encouraged to integrate other means of irrigation planning with soil moisture sensing, such as a push rob to probe the soil profile or OSU Mesonet’s irrigation planner to further validate the sensor data. Further, the cliente should consider their irrigation capacities before investing in soil moisture sensors, as sensors may always show a deficit in low well capacities which cannot meet crop’s water demand.
References:
Taghvaeian, S., D. Porter, J. Aguilar. 20221. Soil moisture-sensing systems for improving irrigation scheduling. BAE-1543. Oklahoma State Cooperative Extension. Available at: https://extension.okstate.edu/fact-sheets/soil-moisture-sensing-systems-for-improving-irrigation-scheduling.html
Datta, S., S. Taghvaeian, J. Stivers. Understanding soil water content and thresholds for irrigation management. BAE-1537. Oklahoma State Cooperative Extension. Available at: https://extension.okstate.edu/fact-sheets/understanding-soil-water-content-and-thresholds-for-irrigation-management.html
For more information please contact Sumit Sharma sumit.sharma@okstate.edu
Cotton disease update: Reniform nematode – 08/25/2025
Maíra Duffeck, OSU Row Crops Extension Pathologist, Department of Entomology and Plant Pathology Oklahoma State University
Maxwell Smith, OSU IPM for Cotton Extension Specialist, Department of Entomology and Plant Pathology, Oklahoma State University
Jenny Dudak, OSU Extension Cotton Specialist, Department of Plant & Soil Sciences Oklahoma State University
Reniform nematode continues to be detected in cotton fields across Oklahoma. During the 2023 and 2024 growing seasons, a survey was conducted in 17 commercial cotton fields located in Tillman, Jackson, Grady, and Caddo counties to assess the presence of parasitic nematodes affecting cotton production. We collected soil samples in areas of the fields showing irregular and stunted cotton plants.
Out of the 17 soil samples collected, reniform nematode was detected in 5 fields, marking the first confirmed report of this pest in Oklahoma cotton. Notably, in one of the positive fields, the reniform nematode population reached 1,569 nematodes per 100 cm³ of soil; more than double the economic threshold of 700 nematodes per 100 cm³. In 2025, a soil sample from a cotton field in Jackson County already tested positive for the reniform nematode.
The reniform nematode, caused by Rotylenchulus reniformis, is one of the most important yield-limiting pathogens of cotton production in the southern U.S. In addition to cotton, the reniform nematode can reproduce on other field crops such as soybean, with yield loss estimates being greater in cotton than soybean. The reniform nematode is easy to introduce into new fields because of its unique ability to survive in a dehydrated state in dry soils. Therefore, it can be transported long distances on field equipment.
We suspect that parasitic nematodes, such as root-knot and reniform nematodes, are already present in many Oklahoma cotton fields, but the damage they cause often goes unnoticed. This is especially important for the reniform nematode, as yield losses can occur without obvious aboveground symptoms. For this reason, monitoring the distribution of this nematode across the cotton fields in Oklahoma is crucial to raise awareness of this emerging issue and to guide future management decisions.
Symptoms and Signs
The expression of symptoms depends on several factors, including the susceptibility of the cotton hybrid, nematode population levels, soil type, and for how long that field has been infested. Affected plants may show reduced growth, delayed flowering, fewer fruits, and smaller fruit size, which together contribute to yield losses in lint or pods. Unlike the southern root-knot nematode (Meloidogyne incognita), the reniform nematode does not induce gall formation on roots, making field diagnosis based solely on visible symptoms challenging. For this reason, soil testing through a nematode assay is often necessary for proper identification. In newly infested fields, stunted plants are typically the most noticeable sign (Figure 1).
Plan of Action
To address this issue, a statewide nematode survey is underway to document the presence, abundance, and geographic distribution of parasitic nematodes in Oklahoma cotton fields. The information generated from this survey will provide a foundation for developing and implementing economically viable strategies to manage this issue and protect cotton production in the state.
How to participate?
Oklahoma cotton growers interested in having their fields tested for parasitic nematodes have several ways to participate in this study:
- Schedule a field visit: Contact Dr. Maira Duffeck to arrange soil sample collection. She can be reached by phone at 347-205-2180 or by email at mairodr@okstate.edu.
- Drop off samples at the Peanut & Cotton Field Day: September 18, 2025, from 5:00–8:00 p.m. at the Caddo Research Station (28054 County Street 2540, Ft. Cobb)
- Drop off samples at the Cotton Field Day: September 25, 2025, from 8:30 a.m. to 1:00 p.m. at the Southwest Research & Extension Center (16721 US Hwy 283, Altus)
More information about dropping off samples on OSU field days can be found on the flyers shown in Figures 2 and 3. Growers can submit soil samples for analysis at no cost, as expenses are covered through a project funded by the Oklahoma Cotton Council in partnership with Cotton Incorporated.
How to collect soil samples for analysis?
- Soil samples should be collected from the root zone of the plants
- Collect 15–20 soil cores (6–8 inches deep) from across the field
- Growers should focus on areas of the field where plants are showing poor growth and development
- Mix the cores thoroughly, then place the mixed soil into a resealable plastic bag
- We need about 2 pints (1 kg) of soil for analysis
- Keep samples cool — store them in a refrigerator until the field day.
- If you collect soil samples from different fields, please label and add field information to the plastic bag accordingly



For Additional Information contact.
Dr. Maíra Duffeck mairodr@okstate.edu
What OK Cotton Growers Should Know about the Two-Spotted Cotton Leafhopper, aka Cotton Jassid
Ashleigh M. Faris, OSU Extension Cropping Systems Entomologist Department of Entomology and Plant Pathology Oklahoma State University
Jenny Dudak, OSU Extension Cotton Specialist, Department of Plant & Soil Sciences Oklahoma State University
Maxwell Smith, OSU IPM for Cotton Extension Specialist Department of Entomology and Plant Pathology, Oklahoma State University
Published 8.29.25
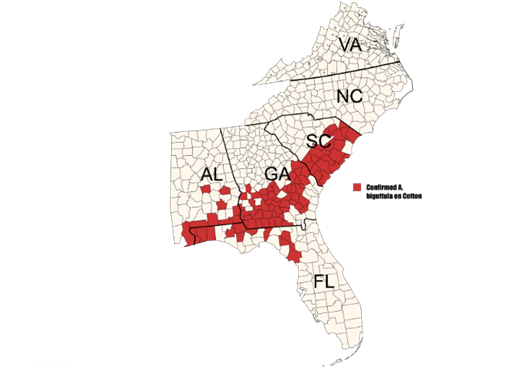
The two-spotted cotton leafhopper, also known as the cotton jassid (Amrasca biguttula), has not been detected in Oklahoma cotton production as of August 27, 2025. However, this insect pest has been confirmed to be causing significant injury in cotton fields that have high populations of the jassid throughout the southeastern United States (Figure 1). Although it is not known to currently be in Oklahoma cotton acres, our cotton growers and consultants are encouraged to keep an eye out for this pest and to report sightings of the insect or related damage to the OSU Cotton Extension team for confirmation.
Two-Spotted Cotton Leafhopper Identification & Injury
Cotton jassids are small, sap-sucking insects that are pale green insects with yellowish-green wings. They can be distinguished from most native North American species by the pair of black spots head and black spots on the tip of each wing (Figure 2). The immature nymph stages are even smaller than the adults; they are wingless but light green in color like the adults. (Figures 3 and 4).



The cotton jassid feeds on the underside of leaves, causing hopperburn—a rapid yellowing, reddening, and browning that can quickly weaken plants (Figure 5). Initially, injury symptoms may look like nutrient deficiency with slight yellowing along the leaf tips and margins. Injury may also present as upward curling/cupping of the leaves. Once early symptoms are visible, leaves decline rapidly, turning red and brown. Because late-stage hopperburn can resemble a spider mite infestation, growers and consultants should scout for the pest causing injury prior to initiating control. Cotton jassid populations and hopperburn symptoms usually begin on field edges before moving into the cotton field.

Suggested Threshold, Scouting, and Chemical Control Guidance
Current recommendations for the suggested threshold and chemical control options are based on insecticide trials conducted by University of Florida, University of Georgia, Clemson University, and the University of Auburn Extension Entomologists. The threshold at which treatment is recommended for the cotton jassid is 2 nymphs per leaf with injury present anywhere in the field. To scout, inspect the underside of the main stem leaf on the third, fourth, or fifth node from the top of the cotton plant. Sample a minimum of 25 leaves across plants and average counts across the number of leaves sampled.
In terms of management, multiple products seem to provide good efficacy. Bidrin (6 oz/a) has been consistent in multiple trials across Florida, Georgia, and South Carlina, but may be difficult if whiteflies are an issue. Centric (2 oz/a) and Transform (1.5 oz/a) seem to be consistent as well. One product that should not be used is Bifenthrin, which has proven not to be effective across multiple trials. Information on suggested chemical control options, their efficacy, and general IPM guidance will be forthcoming as more is learned about this pest in U.S. cotton systems.
If you detect the cotton jassid in Oklahoma cotton, please contact
IPM for Cotton Extension Specialist Maxwell Smith,
Toto, I’ve a feeling we’re not in Kansas anymore. Double Cropping, Orange edition
It has been pointed out that the blog https://osunpk.com/2025/06/09/double-crop-options-after-wheat-ksu-edition/ had a significant Purple Haze. And I should have added the Oklahoma caveat. So Dr. Lofton has provided his take on DC corn in Oklahoma.
Double-crop Corn: An Oklahoma Perspective.
Dr. Josh Lofton, Cropping Systems Specialist.
Several weeks ago, a blog was published discussing double-crop options with a specific focus on Kansas. I wanted to address one part of that blog with a greater focus on Oklahoma, and that section would be the viability of double-crop corn as an option.
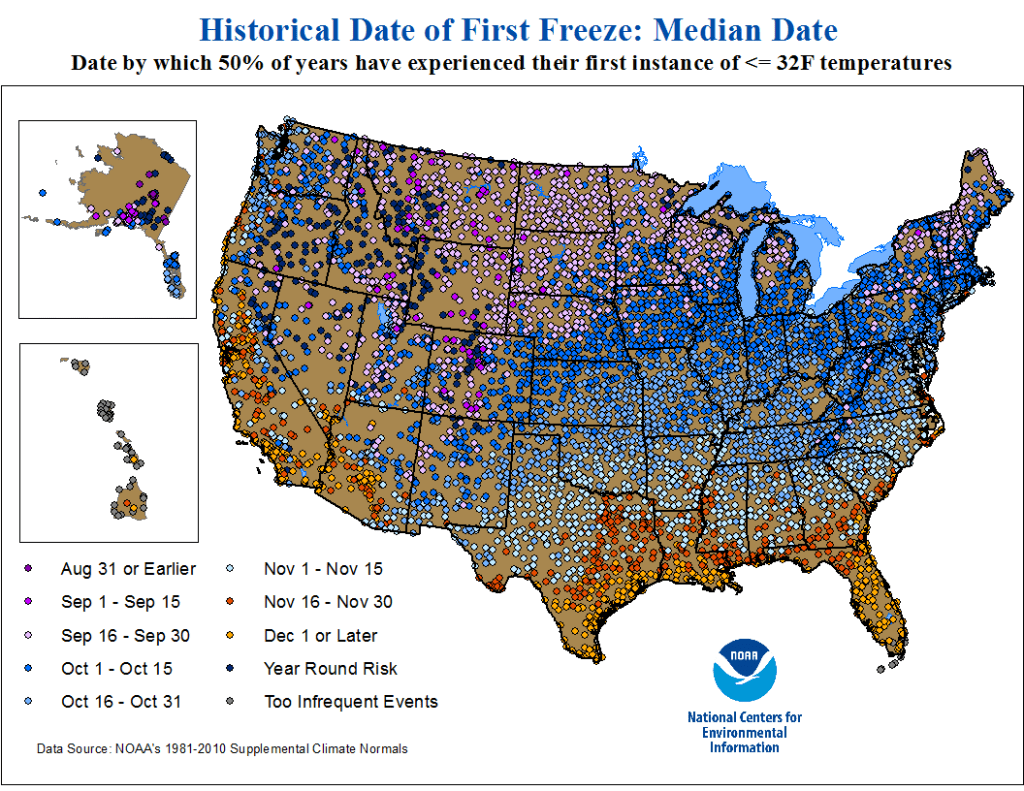
Double-crop farming is considered a high-risk, high-reward system to try. Establishing a crop during the hottest and often driest parts of summer can present challenges that need to be overcome. Double-crop corn faces these same challenges and, in some seasons, even more. However, it is definitely a system that can work in Oklahoma, especially farther south. If you look at that original blog post, one of the main challenges discussed is having enough heat units before the first frost. When examining historic data, like those below from NOAA, the first potential frost date for Northcentral and Northwest Oklahoma may be as early as the first 15 days of October but more often will be in the last 15 days of October. In Southwest and Central Oklahoma, this date shifts even later to the first 15 days of November. This is later than Kansas, especially northern Kansas, which has a much higher chance of experiencing an early October freeze. I do not want to downplay this risk; however, it is one of the biggest risks growers face with this system, and a later fall freeze would greatly benefit it. We have been conducting trials near Stillwater for the past five years on double-crop corn and have only failed the crop once due to an early freeze event. But in that year, both double-crop soybean and sorghum also did not perform well.
The main advantage of double-crop corn is that if you miss the early season window, it offers the best chance for the crop to reach pollination and early grain fill without the stress of the hottest and driest part of the year. Therefore, careful management is crucial to ensure this benefit isn’t lost. In Oklahoma, we have two systems that can support double-crop corn. In more central and southwest Oklahoma, especially under irrigation, farmers can plant corn soon after wheat harvest, similar to other double-crop systems. This planting window helps minimize the impact of Southern Rust, which can significantly reduce yields in some years, and may reduce the need for extensive management. This earlier planting window is often supported by irrigation, enabling the crop to endure the hotter, drier late July and early August periods. Conversely, in northern Oklahoma, planting often occurs in July to allow pollination and grain fill (usually 30-45 days after emergence) to happen in late August and early September. During this period, the chances of rainfall and cooler nighttime temperatures increase, both of which are critical for successful corn production.
Other management considerations include maturity. Based on initial testing in Oklahoma, particularly in the northern areas, we prefer to plant longer-maturity corn. Early corn varieties have a better chance of maturing before a potential early freeze but also carry a higher risk of undergoing critical reproduction stages (pollination and early grain fill) during hot, dry periods in late summer. Testing indicates that corn with a maturity of over 110 days often works well for this. However, this does not mean growers cannot plant shorter-season corn, especially if the season has generally been cooler, though the risk still exists depending on how quickly the crop can grow. Based on testing within the state, the dryland double-crop corn system typically does not require adjustments to other management practices, such as seeding rates or nitrogen application. Because of the need to coordinate leaf architecture and manage limited water resources, higher seeding rates are not recommended. Maintaining current nitrogen levels allows the crop to develop a full canopy.
The final question often comes as; how does it yield? This will depend greatly. Corn looks very good this year across that state, especially what was able to be planted earlier in the spring. However, in recent years, delaying even a couple of weeks beyond traditional planting windows has lowered yields enough that double-crop yields are often similar. We have often harvested between 50-120 bushels per acre in our plots around Stillwater with double-crop systems. So, the yield potential is still there.
In the end, Oklahoma growers know that double-crop is a risk regardless of the crop chosen. There are additional risks for double-crop corn, such as Southern Rust in the south and freeze dates in the north. This risk is increased by the presence of Corn Leaf Aphid and Corn Stunt last season, and it is not clear if these will be ongoing problems. Therefore, growers need to be careful not to expect too much or to invest too heavily in inputs that may not be recoverable if there is a loss. One silver lining is that if double-crop corn doesn’t succeed in any given year, growers can still use it as forage and recover at least some of their costs.
Any questions or concerns reach out to Dr. Lofton: josh.lofton@okstate.edu
Army Worms are Marching!!!!
This article by Brian Pugh (new OSU State Forage Specialist) just came across my desk today in perfect timing as yesterday I saw significant army worm feeding on the crabgrass in my lawn, and not to mention the 20+ caterpillars on my sidewalk. So while Brian is noting Eastern Ok, Id say we are at thresholds in Payne Co also. And no, we don’t need to discuss that my lawn as more crabgrass than Bermuda.
Fall Armyworms Have Arrived In Oklahoma Pastures and Hayfields
Brian C. Pugh, Forage Extension Specialist
Fall armyworms (FAW) are caterpillars that directly damage Bermudagrass and other introduced forage pastures, seedling wheat, soybean and residential lawns. There have been widespread reports of FAW buildups across East Central and Northeast Oklahoma in the first two weeks of July. Current locations exceeding thresholds for control are Pittsburg, McIntosh and Rogers counties.

Female FAW moths lay up to 1000 eggs over several nights on grasses or other plants. Within a few days, the eggs hatch and the caterpillars begin feeding. Caterpillars molt six times before becoming mature, increasing in size after each molt (instars). The first instar is the caterpillar just after it hatches. A second instar is the caterpillar after it has shed its skin for the first time. A sixth instar has shed its skin five times and will feed, bury itself in the soil, and pupate. The adult moth will emerge from the pupa in two weeks and begin the egg laying process again after a suitable host plant is found. Newly hatched larvae are white, yellow, or light green and darken as they mature. Mature FAW measure 1½ inches long with a body color that ranges from green, to brown to black.

Large variation in color is normal and shouldn’t be used alone as an identifying characteristic. They can most accurately be distinguished by the presence of a prominent inverted white “y” on their head. However, infestations need to be detected long before they become large caterpillars. Small larvae do not eat through the leaf tissue, but instead, scrape off all the green tissue and leave a clear membrane that gives the leaf a “window pane” appearance. Larger larvae however, feed voraciously and can completely consume leaf tissue.

FAW are “selective grazers” and tend to select the most palatable species of forages on any given site to lay eggs for young larvae to begin feeding. The caterpillars also tend to feed on the upper parts of the plant first which are younger and lower in fiber content. Forage stands that are lush due to fertility applications are often attacked first and should be scouted more frequently.

To scout for FAW, plants from several locations within the field or pasture need to be examined. Examine plants along the field margin as well as in the interior. Look for “window paned” leaves and count all sizes of larvae. OSU suggests a treatment threshold is two or three ½ inch-long larvae per linear foot in wheat and three or four ½ inch-long larvae per square foot in pasture. An easy-to-use scouting aid can be made for pasture by bending a wire coat hanger into a hoop and counting FAW in the hoop. The hoop covers about 2/3 of a square foot, so a threshold in pasture would be an average of two or three ½ inch-long larvae per hoop sample. An excellent indicator plant in forage stands is Broadleaf Signalgrass (seen in the foreground of the hay bale picture). Broadleaf Signalgrass tends to be preferentially selected by female moths and is one of the first species that window paned tissue is observed during the onset of an infestation.
Approximately 70% of the forage consumed during an armyworm’s lifetime occurs in the final instar before pupating into a moth. This indicates that control measures should focus on small instar caterpillars (1/2 inch or less) before forage loss increases exponentially. Additionally, small larvae are much more susceptible to insecticide control than larger caterpillars.

Remember, FAW are actively reproducing up until a good killing frost, so don’t let your guard down. If you think you have an infestation of fall armyworm please contact your local County Extension Educator. Additionally, before considering chemical control consult your Educator for insecticide recommendations labeled for forage use.
For more information or insecticide options consult:
Oklahoma State University factsheet:
CR-7193, Management of Insect Pests in Rangeland and Pasture
https://extension.okstate.edu/fact-sheets/management-of-insect-pests-in-rangeland-and-pasture.html
Double Crop Options After Wheat (KSU Edition)
Stolen from the KSU e-Update June 5th 2025.
Double cropping after wheat harvest can be a high-risk venture for grain crops. The remaining growing season is relatively short. Hot and/or dry conditions in July and August may cause problems with germination, emergence, seed set, or grain fill. Ample soil moisture this year can aid in establishing a successful crop after wheat harvest. Double-cropping forages after wheat works well even in drier regions of the state.
The most common double crop grain options are soybean, sorghum, and sunflower. Other possibilities include summer annual forages and specialized crops such as proso millet or other short-season summer crops, even corn. Cover crops are also an option for planting after wheat (see the companion eUpdate article “Cover crops grown post-wheat for forage”).
Be aware of herbicide carryover potential
One major planting consideration after wheat is the potential for herbicide carryover. Many herbicides applied to wheat are Group 2 herbicides in the sulfonylurea family with the potential to remain in the soil after harvest. If a herbicide such as chlorsulfuron (Glean, Finesse, others) or metsulfuron (Ally) has been used, then the most tolerant double crop will be sulfonylurea-resistant varieties of soybean (STS, SR, Bolt) or other crops. When choosing to use herbicide-resistant varieties, be sure to match the resistance trait with the specific herbicide (not only the herbicide group) that you used. This is especially true when looking at sunflowers as a double crop. There are sunflowers with the Clearfield trait, which allows Beyond herbicide applications, and ExpressSun sunflowers, which allow an application of Express herbicide. While both of these herbicides are Group 2 (ALS-inhibiting herbicides), the Clearfield trait and ExpressSun are not interchangeable, and plant damage can result from other Group 2 herbicides.
Less information is available regarding the herbicide carryover potential of wheat herbicides to cover crops. There is little or no mention of rotational restrictions for specific cover crops on the labels of most herbicides. However, this does not mean there are no restrictions. Generally, there will be a statement that indicates “no other crops” should be planted for a specified amount of time, or that a bioassay must be conducted prior to planting the crop.
Burndown of summer annual weeds present at planting is essential for successful double-cropping. Assuming glyphosate-resistant kochia and pigweeds are present, combinations of glyphosate with products such as saflufenacil (Sharpen) or tiafenacil (Reviton), or alternative treatments such as paraquat may be required. Dicamba or 2,4-D may also be considered if the soybean varieties with appropriate herbicide resistance traits are planted. In addition, residual herbicides for the double crop should be applied at this time.
Management, production costs, and yield outlooks for double crop options are discussed below.
Soybeans
Soybeans are likely the most commonly used crop for double cropping, especially in central and eastern Kansas (Figure 1). With glyphosate-resistant varieties, often the only production cost for planting double crop soybeans was the seed, an application of glyphosate, and the fuel and equipment costs associated with planting, spraying, and harvesting. However, the spread of herbicide-resistant weeds means additional herbicides will be required to achieve acceptable control and minimize the risk of further development of resistant weeds.

Weed control. The weed control cost cannot really be counted against the soybeans, since that cost should occur whether or not a soybean crop is present. In fact, having soybeans on the field may reduce herbicide costs compared to leaving the field fallow. Still, it is recommended to apply a pre-emergence residual herbicide before or at planting time. Later in the summer, a healthy soybean canopy may suppress weeds enough that a late-summer post-emergence application may not be needed.
Variety selection for double cropping is important. Soybeans flower in response to a combination of temperature and day length, so shifting to an earlier-maturing variety when planting late in a double crop situation will result in very short plants with pods that are close to the ground. Planting a variety with the same or perhaps even slightly later maturity rating (compared to soybeans planted at a typical planting date) will allow the plant to develop a larger canopy before flowering. Planting a variety that is too much later in maturity, however, increases the risk that the beans may not mature before frost, especially if long periods of drought slow growth. The goal is to maximize the length of the growing season of the crop, so prompt planting after wheat harvest time is critical. The earlier you can plant, the higher the yield potential of the crop if moisture is not a limiting factor.
Fertilizer considerations. Adding some nitrogen (N) to double-crop soybeans may be beneficial if the previous wheat yield was high and the soil N was depleted. A soil test before wheat harvest for N levels is recommended. Use no more than 30 lbs/acre of N. It would be ideal to knife-in the fertilizer. If that is not possible, banding it on the soil surface would be acceptable. Do not apply N in the furrow with soybean seed as severe stand loss can occur.
Seeding rates and row spacing. Seeding rate can be slightly increased if soybeans are planted too late in order to increase canopy development. Narrow row spacing (15-inch or less) has often resulted in a yield advantage compared to 30-inch rows in late plantings. Soybeans planted in narrow rows will canopy over more quickly than in wide rows, which is important when the length of the growing season is shortened. Narrow rows also offer the benefits of increasing early-season light capture, suppressing weeds, and reducing erosion. On the other hand, the advantage of planting in wide rows is that the bottom pods will usually be slightly higher off the soil surface to aid harvest. The other consideration is planting equipment. Often, no-till planters will handle wheat residue better and place seeds more precisely than drills, although the difference has narrowed in recent years.
What are typical yield expectations for double-crop soybeans? It varies considerably depending on moisture and temperature, but yields are usually several bushels less than full-season soybeans. A long-term average of 20 bushels per acre is often mentioned when discussing double-crop soybeans in central and northeast Kansas. Rainfall amount and distribution can cause a wide variation in yields from year to year. Double-crop soybean yields typically are much better as you move farther southeast in Kansas, often ranging from 20 to 40 bushels per acre.
A recent publication explores the potential yield of double-crop soybeans relative to full-season yield (Figure 2) and the most limiting factors affecting the yields for double-crop soybeans. The link to this article is: https://bookstore.ksre.ksu.edu/pubs/MF3461.pdf.
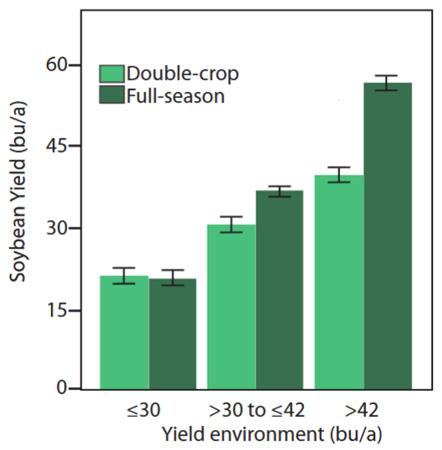
Grain Sorghum
Grain sorghum is another double crop option. Unlike soybeans, sorghum hybrids for double cropping should be earlier maturing hybrids. Sorghum development is primarily driven by the accumulation of heat units, and the double crop growing season is too short to allow medium-late or late hybrids to mature before the first frost in most of Kansas.
Seeding rates and row spacing. Late-planted sorghum likely will not tiller as much as early plantings and can benefit from slightly higher seeding rates than would be used for sorghum planted at an earlier date. Narrow row spacing is advised, especially if the outlook for rainfall is good.
Fertilizer considerations. A key component for the estimation of N application rates is the yield potential. This will largely determine the N needs. It is also important to consider potential residual N from the wheat crop. This can be particularly important when wheat yields are lower than expected. In that situation, additional available N may be present in the soil. Assess the amount of profile N by taking soil samples at a depth of 24 inches and submitting them for analysis at a soil testing laboratory.
Double crop sorghum planted into average or greater-than-average amounts of wheat residue can result in a challenging amount of residue to deal with when planting next year’s crop. Nitrogen fertilizer can be tied up by wheat residue, so use application methods to minimize tie-up, such as knifing into the soil below the residue.
Weed control. Weed control can be important in double-crop sorghum. Warm-season annual grasses, such as crabgrass, can reduce double-crop sorghum yields. Using a chloroacetamide-and-atrazine pre-emergence product may be key to successful double-crop sorghum production. Herbicide-resistant grain sorghum varieties will allow the use of imazamox (Imiflex in igrowth sorghums) or quizalofop (FirstAct in DoubleTeam grain sorghum) that can control summer annual grasses.
No-till studies at Hesston documented 4-year average double crop sorghum yields of 75 bushels per acre compared to about 90 bushels per acre for full-season sorghum. A different 10-year study that did not have double crop planting but did compare early- and late-planting dates averaged 73 bushels per acre for May planting vs. 68 bushels per acre for June planting.
Sunflowers
Sunflowers can be a successful double crop option anywhere in the state, provided there is enough moisture at planting time to get a stand. Sunflowers need more moisture than any other crop to germinate and emerge because of the large seed. Therefore, stand establishment is important. Planting immediately after wheat harvest on a limited irrigation field can be a good fit to help with stand establishment.
Seeding rates and hybrid selection. When double-cropping sunflowers, producers should use similar seeding rates to what is typical for the area for full-season sunflowers. While full-season sunflowers can be successful in double-crop production, utilizing shorter-season hybrids can increase the likelihood of the sunflowers blooming and maturing before a killing frost.
Weed control. First, it is important to check the herbicide applications on the wheat. The rotation restriction to sunflowers after several commonly used wheat herbicides is 22-24 months.
Weed control can be an issue with double-crop sunflowers since herbicide options are limited, especially post-emergence. Thus, controlling weeds prior to sunflower planting is critical and may be complicated pre-plant restrictions for some herbicides. Planting Clearfield or ExpressSun sunflowers will provide additional post-emergence herbicide options, but ALS-resistant kochia and pigweeds still won’t be controlled. Imazamox (Beyond in Clearfield sunflower) has activity on small annual grasses as well as many broadleaf weeds, if they are not ALS-resistant.
Summer annual forages
With mid-July plantings, and where herbicide carryover issues are not a concern, summer annual sorghum-type forages are also a good double crop option. A study planted July 21, 2008 near Holton, when summer rainfall was very favorable, provided yields of 2.5 to 3 tons dry matter/acre for hybrid pearl millet and sudangrass at the low end to 4 to 5 tons dry matter/acre for forage sorghum, BMR forage sorghum, photoperiod sensitive forage sorghum, and sorghum x sudangrass hybrids. Earlier plantings may produce even more tonnage, as long as there is adequate August rainfall.
One challenge with late-planted summer annual forages is getting them to dry down when harvest is delayed until mid- to late-September. Wrapping bales or bagging to make silage are good ways to deal with the higher moisture forage this late in the year.
Corn
Is double-crop corn a viable option? Corn is typically not recommended for late June or July plantings because yield is usually substantially less than when planted earlier.
Typically, mid-July planted corn struggles during pollination and seldom receives sufficient heat units to fill grain before frost. Very short-season corn hybrids (80 to 95 RM) have the greatest chance of maturing before frost in double crop plantings, but generally have less yield potential when compared to hybrids of 100 RM or more used for full-season plantings. Short-season hybrids often set the ear fairly close to the ground, increasing the harvest difficulty. Glyphosate-resistant hybrids will make weed control easier with double crop corn, but problems remain present with late-emerging summer weeds such as pigweeds, velvetleaf, and large crabgrass. Keep in mind, corn is very susceptible to carryover of most residual ALS herbicides used in wheat.
Considerations for altering seeding rates and variety/hybrid maturity for the crops discussed above are summarized in Table 1.
Table 1. Seeding rate and variety/hybrid relative maturity considerations for double crops compared to full-season.
| Crop | Seeding rate | Relative maturity |
| ???????? Difference between double crop and full-season ???????? | ||
| Soybean | Increase | No change or longer |
| Sorghum | Increase | Shorter |
| Sunflower | No change | Shorter |
| Corn | No change | Shorter |
Volunteer wheat control
One of the issues with double cropping that is often overlooked by producers is the potential for volunteer wheat in the crop following wheat. If volunteer wheat emerges and goes uncontrolled, it can cause serious problems for nearby wheat fields in the fall as a host for the wheat streak mosaic complex of viruses [wheat streak mosaic (WSMV), High Plains disease (HPD), and triticum mosaic (TriMV)] that are transmitted by the wheat curl mite (WCM).
Volunteer wheat can generally be controlled fairly well with glyphosate or Group 1 herbicides such as quizalofop (Assure II, others), clethodim (Select Max, others), or sethodydim (Poast Plus, others), but control is reduced during times of drought stress. Atrazine can provide control of volunteer wheat in double-crop corn or sorghum, but control can be erratic depending on rainfall patterns.
For more detailed information about herbicides, see the “2025 Chemical Weed Control for Field Crops, Pastures, and Noncropland” guide available online at https://www.bookstore.ksre.ksu.edu/pubs/CHEMWEEDGUIDE.pdf or check with your local K-State Research and Extension office for a paper copy. The use of trade names is for clarity to readers and does not imply endorsement of a particular product, nor does exclusion imply non-approval. Always consult the herbicide label for the most current use requirements.
To Subscribe to the KSU Agronomy E-Updates follow this link
https://eupdate.agronomy.ksu.edu/index_new_prep.php
Authors contributing to the post
Sarah Lancaster, Weed Management Specialist
slancaster@ksu.edu
John Holman, Cropping Systems Agronomist
jholman@ksu.edu
Logan Simon, Southwest Area Agronomist
lsimon@ksu.edu
Tina Sullivan, Northeast Area Agronomist
tsullivan@ksu.edu
Jeanne Falk Jones, Multi-County Agronomist
jfalkjones@ksu.edu
Meet the Aster Leafhopper and Learn How to Distinguish it from the Corn Leafhopper
Ashleigh M. Faris: Extension Cropping Systems Entomologist, IPM Coordinator
Release Date June 3 2025
Last year’s corn stunt disease outbreak, caused by the corn leafhopper transmitting pathogens associated with corn stunt disease, has been on everyone’s minds. Over the past few weeks, I’ve received several calls from growers, crop consultants, and industry partners concerned about leafhoppers in corn. Fortunately, none have been corn leafhoppers, the vast majority have instead been aster leafhoppers. So far, no corn leafhoppers have been reported north of central Texas. Oklahoma did not have any reports of overwintering corn leafhoppers so if we have the insect this year it will need to migrate northward from where it currently resides. For a refresher on the corn leafhopper and corn stunt disease, check out these two previously posted OSU Pest e-Alerts: EPP-25-3and EPP-23-17.
Leafhoppers in general are insects that we have had for many years in our row and field crops. But we likely did not pay attention to them or notice them until this past year due to our heightened awareness of their existence thanks to the corn leafhopper and corn stunt disease. Below is guidance on how to distinguish between the corn leafhopper and aster leafhopper. Remember, if the corn leafhopper is detected in the state, OSU Extension will notify growers, consultants, and industry partners through Pest e-Alerts and our social media channels.
Aster Leafhopper Overview
The aster leafhopper (aka six spotted leafhopper), Macrosteles quadrilineatus, is native to North America and can be found in every U.S. state, as well as Canada. This polyphagous insect feeds on over 300 host plant species including weeds, vegetables, and cereals. Like many other leafhoppers, the aster leafhopper can be a vector of pathogens that cause disease, but corn stunt is not one of them. Instead, aster leafhoppers cause problems in traditional vegetable growing operations, as well as floral production. There is currently no concern for this insect being a vector of disease in row or field crops, including corn. Check out the OSU Pest a-Alert EPP-23-1to learn more about this insect and aster yellows disease.
Aster Leafhopper versus Corn Leafhopper
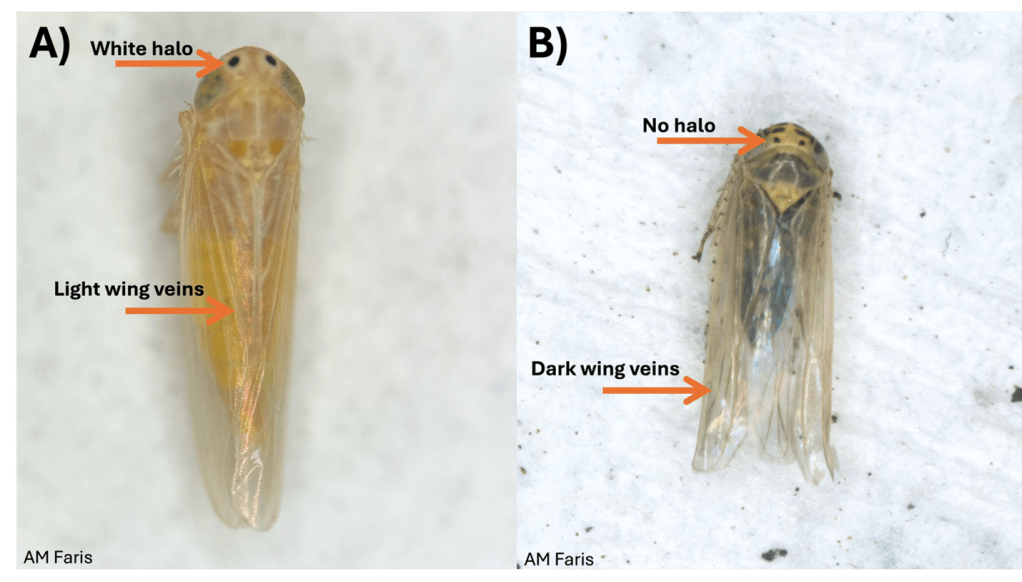
The corn leafhopper (Photo 1A) and the aster leafhopper, as well as many other leafhopper species have two black dots located between the eyes of the insect (Photo 1). Aster leafhopper adults are 0.125 inches (3 mm) long, with transparent wings that bear strong veins, and darkly colored abdomens (Photo 1B). Their dark abdomen can cause the aster leafhopper to appear grey when you see them in the field. Their long wings can also make the insect appear to have a similar appearance to the corn leafhopper (Dalbulus maidis) (Photo 1).
Characteristics that differentiate the corn leafhopper from the aster leafhopper are as follows. When viewed from above (dorsally): 1) the corn leafhopper’s dots between the eyes have a white halo around them and the aster leafhopper’s dots between eyes lack the white halo and 2) the corn leafhopper has lighter/finer wing veination than the aster leafhopper (Photo 1). When when viewed from their underside (ventrally) 3) the corn leafhopper lacks markings on their face whereas the aster leafhopper has lines/spot on the face and 4) the abdomen of the corn leafhopper lacks the dark coloration of the aster leafhopper (Photo 2).

Confirming Corn Leafhopper Identification
It is important to note that many insects will have their cuticle darken as they age. This, along with there being light and dark morphs of many insects can lend to additional confusion when distinguishing one species from another. If you believe that you have a corn leafhopper then you need to collect the insect and send it to a trained entomologist that can verify the identity of the insect under the microscope. Leafhoppers in general are fast moving insects but they can be collected in an insect net or using a handheld vacuum (see EPP-25-3). You can submit samples to the OSU Plant Disease and Insect Diagnostic Lab.
Please feel free to reach out to OSU Cropping Systems Extension Entomologist Dr. Ashleigh Faris with any questions or concerns. @ ashleigh.faris@okstate.edu
PRE-EMERGENT RESIDUAL HERBICIDE ACTIVITY ON SOYBEANS, 2025
Liberty Galvin, Weed Science Specialist
Karina Beneton, Weed Science Graduate Student.
Objective
Determine the duration of residual weed control in soybean systems following the application of Preemergent (PRE) herbicides when applied alone and in tank-mix combination.
Why we are doing the research
PRE herbicides offer an effective means of suppressing early-season weed emergence, thereby minimizing competition during the critical early growth stage. However, evolving herbicide resistance and the need for longer-lasting weed suppression underscore the importance of evaluating multiple modes of action and their residual properties alone and tank-mixed.
Field application experimental design and methods
Field experiments were conducted in 2022, 2023, and 2024 growing seasons in Bixby, Lane, and Ft. Cobb FRSU Research Stations across Oklahoma. Each herbicide (listed in Table 1) was tested individually, in 2-way combinations, 3-way mixtures, and finally as 4-way combinations that included all active ingredients listed at the label rate.
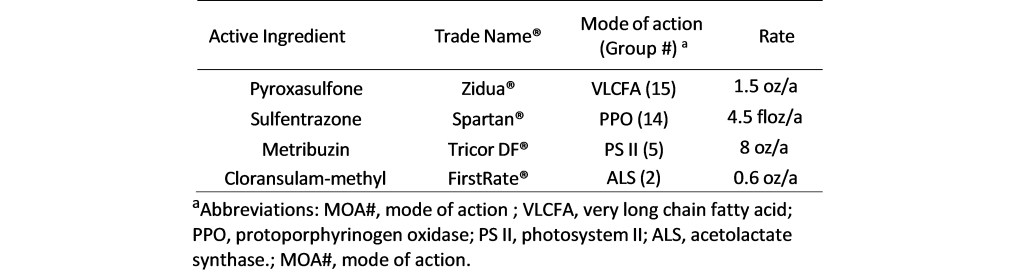
Soybeans were planted at rates between 116,000 and 139,000 seeds/acre from late May to early June, depending on the year and location. The variety used belongs to the indeterminate mid- maturity group IV, with traits conferring tolerance to glyphosate (group 9 mode of action), glufosinate (group 10), and dicamba (group 4). Not all soybean varieties have metribuzin tolerance. Please read the herbicide label and consult your seed dealer for acquiring tolerant varieties. Row spacing was 76 cm at Bixby and Lane, and 91 cm at Fort Cobb. PRE treatments were applied immediately after planting at each experimental location.
POST applications consisted of a tank-mix of dicamba (XtendiMax VG® – 22 floz/acre), glyphosate (Roundup PowerMax 3®- 30 floz/acre), S-metolachlor (Dual II Magnum® – 16 floz/acre), and potassium carbonate (Sentris® – 18 floz/acre). Applications were made on different dates, mostly after the first 3 weeks following PRE treatments. These timings were based on visual weed control ratings, particularly for herbicides applied alone or in 2-way combinations, which showed less than 80% control at those early evaluation dates. The need for POST applications also depended on the species present at each site, with most fields being dominated by pigweed, as illustrated in the figure below.
Results
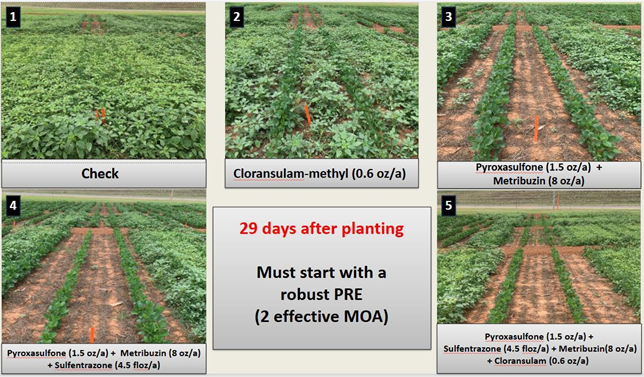
Tank-mixed PRE herbicide combinations generally provided superior residual control compared to a single mode of action application (Shown in Figure 1). Timely post-emergent (POST) herbicide applications helped sustain high levels of weed suppression, particularly as the effectiveness of residual PRE declined.
Residual control of tank-mixed PRE
Some herbicides applied alone or in simple 2-way mixes, such as sulfentrazone + chloransulam- methyl and pyroxasulfone + chloransulam-methyl required POST applications within 20 to 29 days after PRE, indicating moderate residual control.
In contrast, 2-way combinations containing metribuzin, such as sulfentrazone + metribuzin and pyroxasulfone + metribuzin, extended control up to 50 days after PRE in some cases, highlighting metribuzin’s importance even in less complex formulations.
Furthermore, 3-way and 4-way combinations including metribuzin provided the longest-lasting control, delaying POST applications up to 51–55 days after PRE.
Injury of specific weeds
Palmer amaranth (Amaranthus palmeri) control in Bixby was consistently high (≥90%) at 2 weeks after PRE in 2022 and 2024 across all treatments. At 4 WAPRE, treatments containing metribuzin alone or in combination maintained strong control (90% or greater).
Texas millet (i.e., panicum; Urochloa texana) and large crabgrass (Digitaria sanguinalis) were effectively managed with most treatments delivering over 90% control early in the season and maintaining performance throughout. In 2024, control remained generally effective, though pyroxasulfone alone showed a temporary lack of control for Texas millet, and single applications declined in effectiveness against large crabgrass later in the season. These reductions were likely due to continuous emergence and the natural decline in residual herbicide activity due to weather. The most consistent late-season control for both species came from 3- and 4-way herbicide combinations.
Morningglory (Ipomoea purpurea) control reached full effectiveness (100%) only when POST herbicides were applied, across all years and locations. Their late emergence beyond the residual window of PRE herbicides reinforces the importance of sequential herbicide applications for season-long control.
Take home messages:
- Incorporating PRE and POST herbicides slows the rate of herbicide resistance
- Tank mixing with *different modes of action* ensures greater weed control by having activity on multiple metabolic pathways within the plant.
- Tank mixing with PRE herbicides could reduce the number of POST applications required, and
- Provides POST application flexibility due to residual of PRE application
For additional information, please contact Liberty Galvin at 405-334-7676 | LBGALVIN@OKSTATE.EDU or your Area Agronomist extension specialist.