Home » Posts tagged 'forage'
Tag Archives: forage
Army Worms are Marching!!!!
This article by Brian Pugh (new OSU State Forage Specialist) just came across my desk today in perfect timing as yesterday I saw significant army worm feeding on the crabgrass in my lawn, and not to mention the 20+ caterpillars on my sidewalk. So while Brian is noting Eastern Ok, Id say we are at thresholds in Payne Co also. And no, we don’t need to discuss that my lawn as more crabgrass than Bermuda.
Fall Armyworms Have Arrived In Oklahoma Pastures and Hayfields
Brian C. Pugh, Forage Extension Specialist
Fall armyworms (FAW) are caterpillars that directly damage Bermudagrass and other introduced forage pastures, seedling wheat, soybean and residential lawns. There have been widespread reports of FAW buildups across East Central and Northeast Oklahoma in the first two weeks of July. Current locations exceeding thresholds for control are Pittsburg, McIntosh and Rogers counties.

Female FAW moths lay up to 1000 eggs over several nights on grasses or other plants. Within a few days, the eggs hatch and the caterpillars begin feeding. Caterpillars molt six times before becoming mature, increasing in size after each molt (instars). The first instar is the caterpillar just after it hatches. A second instar is the caterpillar after it has shed its skin for the first time. A sixth instar has shed its skin five times and will feed, bury itself in the soil, and pupate. The adult moth will emerge from the pupa in two weeks and begin the egg laying process again after a suitable host plant is found. Newly hatched larvae are white, yellow, or light green and darken as they mature. Mature FAW measure 1½ inches long with a body color that ranges from green, to brown to black.

Large variation in color is normal and shouldn’t be used alone as an identifying characteristic. They can most accurately be distinguished by the presence of a prominent inverted white “y” on their head. However, infestations need to be detected long before they become large caterpillars. Small larvae do not eat through the leaf tissue, but instead, scrape off all the green tissue and leave a clear membrane that gives the leaf a “window pane” appearance. Larger larvae however, feed voraciously and can completely consume leaf tissue.

FAW are “selective grazers” and tend to select the most palatable species of forages on any given site to lay eggs for young larvae to begin feeding. The caterpillars also tend to feed on the upper parts of the plant first which are younger and lower in fiber content. Forage stands that are lush due to fertility applications are often attacked first and should be scouted more frequently.

To scout for FAW, plants from several locations within the field or pasture need to be examined. Examine plants along the field margin as well as in the interior. Look for “window paned” leaves and count all sizes of larvae. OSU suggests a treatment threshold is two or three ½ inch-long larvae per linear foot in wheat and three or four ½ inch-long larvae per square foot in pasture. An easy-to-use scouting aid can be made for pasture by bending a wire coat hanger into a hoop and counting FAW in the hoop. The hoop covers about 2/3 of a square foot, so a threshold in pasture would be an average of two or three ½ inch-long larvae per hoop sample. An excellent indicator plant in forage stands is Broadleaf Signalgrass (seen in the foreground of the hay bale picture). Broadleaf Signalgrass tends to be preferentially selected by female moths and is one of the first species that window paned tissue is observed during the onset of an infestation.
Approximately 70% of the forage consumed during an armyworm’s lifetime occurs in the final instar before pupating into a moth. This indicates that control measures should focus on small instar caterpillars (1/2 inch or less) before forage loss increases exponentially. Additionally, small larvae are much more susceptible to insecticide control than larger caterpillars.

Remember, FAW are actively reproducing up until a good killing frost, so don’t let your guard down. If you think you have an infestation of fall armyworm please contact your local County Extension Educator. Additionally, before considering chemical control consult your Educator for insecticide recommendations labeled for forage use.
For more information or insecticide options consult:
Oklahoma State University factsheet:
CR-7193, Management of Insect Pests in Rangeland and Pasture
https://extension.okstate.edu/fact-sheets/management-of-insect-pests-in-rangeland-and-pasture.html
Nitrogen rate and timing for a forage wheat crop. Year 1 Results.
Written by
Mr. Bronc Finch, PhD. Student, Precision Nutrient Management.
Dr. Brian Arnall, Precision Nutrient Management Extension Specialist.
In cooperation with Dr. James Rogers, Noble Research Institute.
With the amount of wheat acreage in Oklahoma being utilized for grazing cattle, and much of that land grazed completely instead of harvested for grain, many questions have arose regarding the management of grazed cropland. A major question in the management of a graze-out wheat crop pertains to fertilizer management strategies. A study developed in co-operation with the Noble Research Institute is attempting to answer these questions among others. In 2019 the trial was established at three locations: near Lake Carl Blackwell in Stillwater, OSU South Central Research Station in Chickasha, and Noble Research Dupy farm in Gene Aurty, Oklahoma. Each of these three sites were setup with three nitrogen (N) treatments in Gallagher winter wheat, with 2 pre-plant applications of 60 and 120 pounds per acre, and a 60 pound pre-plant and 60 pound top-dress application. Grazing simulation harvests were taken at two times with the top-dress N being applied after regrowth was noticed following the winter season. The Dupy location was planted late and therefore only had a single harvest at the end of the season. Rising plate meter measurement were collected at feekes 7.5 and represented in the graphs below as Mid-season. The Chickasha location revealed unexpectedly high residual soil N levels, which resulted in no differences in dry matter biomass for the first harvest, which was delayed until early march due to excessive rains. The second harvest at Chickasha did show treatment differences with a 0.4 ton difference between the 60 and 120 lbs preplant N rates and increase of 0.8 ton increase over the 120 lb pre-plant when the additional 60 lbs of N was delayed. LCB had a timely first harvest in December resulting in the 120 lb N application outperforming the 60lb N applications by ≥0.33 tons. The second harvest further showed how the split application of N proves beneficial for biomass production. As the split application increased yields by 1.7 and 2.6 tons over the 120 lb and 60 lb preplant applications, respectively. The Dupy location revealed no significant difference in dry matter biomass yield between N treatments at the time of the rising plate meter measurements or for the final cutting.
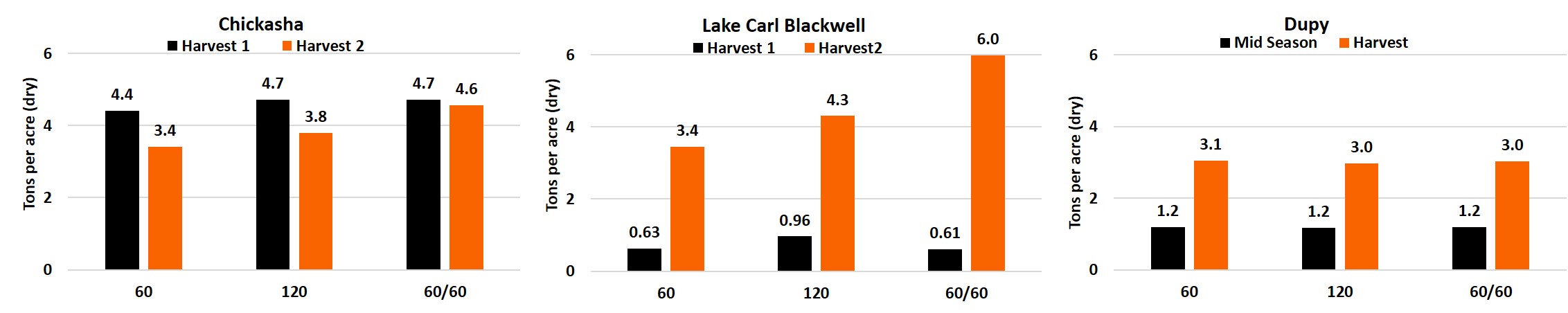
Figure 1. Dry matter harvest results for each of the harvest dates from the graze out wheat trials from the Chickasha, Lake Carl Blackwell, and Dupy locations for three fertilizer treatments. 60: 60 lbs of nitrogen applied preplant, 120: 120 lbs of nitrogen applied preplant, 60/60: Split application 60 lbs of nitrogen preplant and 60 lbs applied top-dress. Dupy only had one harvest date, the Mid-season yield is estimated via rise-plate measurements taken at Feekes 7.5.
The Chickasha and Lake Carl Blackwell (LCB) locations produced an increase in total yield with both the increase of applied N and the split application of N. The 60 lb increase in applied N at preplant, 60 lbs vs 120 lbs, produced a 0.7 and 1.2 ton increase in total dry matter harvested at Chickasha and LCB, respectively. As expected an increase in N increased the yield of wheat biomass for grazing production. The top-dress application, which was made as a late season post Feekes 6 (hollow stem), produced more biomass for graze-out wheat production. The split application of 60 lbs of N preplant and 60 lbs of N top-dress increased dry matter by .8 and 1.3 tons over 120 lbs applied preplant at Chickasha and LCB, respectively. Chickasha yielded higher biomass production than the LCB location due to increased residual N.

Figure 2. Total dry matter harvest results for the graze out wheat trials from the Chickasha, Lake Carl Blackwell (LCB), and Dupy locations for three fertilizer treatments. 60: 60 lbs of nitrogen applied preplant, 120: 120 lbs of nitrogen applied preplant, 60/60: Split application 60 lbs of nitrogen preplant and 60 lbs applied top-dress.
For the following discussion remember that protein is determined by N concentration, so that a increase in N uptake is the same as an increase in protein. Evaluation of the N uptake (% N in the biomass x amount of biomass harvested) over the season revealed treatment effects at all locations, which was not seen from biomass yield. Chickasha and LCB revealed a 20% or greater increase in N uptake with the 120 lb application over the 60 lb application of N at pre-plant. The late season top-dress application yielded a 3, 27, and 27 percent increase in uptake for Chickasha, LCB, and Dupy locations, respectively, over the 120 lb pre-plant application. Although, these results are expected from these results, there are a few things we did not expect. The 120 lb N application did not increase the N uptake above that of the 60 lb application. However, the split application of N resulted in an additional >40 lbs uptake, aka increased protein.
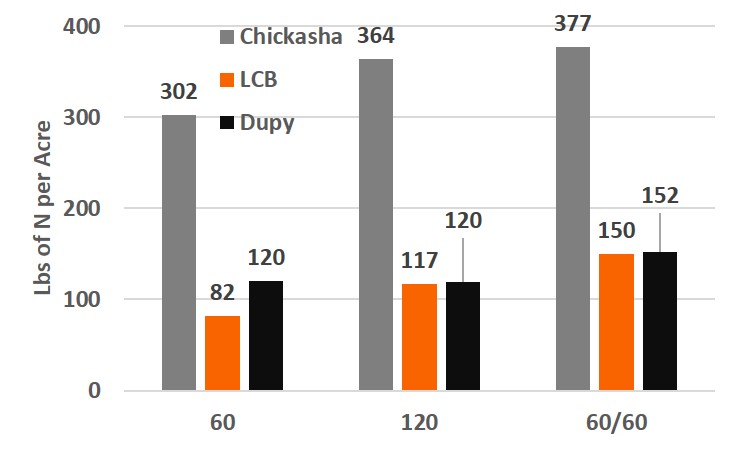
Figure 3. Total nitrogen uptake results for the graze out wheat trials from the Chickasha, Lake Carl Blackwell, and Dupy locations for three fertilizer treatments. 60: 60 lbs of nitrogen applied preplant, 120: 120 lbs of nitrogen applied preplant, 60/60: Split application 60 lbs of nitrogen preplant and 60 lbs applied top-dress.
This study also includes summer forages with and without additional fertilizer. The study will be continued for multiple years on the same locations to evaluate the impact of management on production and soil characteristics. But one surprising note has already been made, in all three locations a greatly delay top-dress still increased N-uptake. In two location it significantly increase yield and protein. This data is falling in line with the grain only data (How late can you wait) showing that an application of N at Feekes 6 (Hollow stem) and even shortly after can provide positive return on investments.
For any questions for comments please contact
Brian Arnall
b.arnall@okstate.edu
405-744-1722
Watch Forage Nitrate Closely on Certain Crops
Nitrate is one of the major nitrogen (N) forms utilized by plants. Excessive nitrate accumulation can occur when the uptake of nitrate exceeds its utilization in plants for protein synthesis due to factors such as over N fertilization and stressful weather conditions. It can be toxic to livestock when too much nitrate is accumulated in the forage crops. Sorghum and millet have been noted as having a high potential for accumulating nitrate. Producers should watch their forage nitrate closely to avoid cattle fatality and to better manage their hay crop since we have seen many high nitrate forage samples every year. Normally, drought stress, cloudy weather and other climatic conditions will enhance nitrate accumulation in the plant. In addition, forage planted in failed wheat fields with high soil residual nitrogen unused by wheat can result in high forage nitrate problem too.

Figure 1. Summary of our laboratory nitrate test results in the past on two major warm season forage crops.
It is considered potentially toxic for all cattle when nitrate in the forage is greater than 10,000 ppm. Producers should avoid grazing or feeding with high nitrate hays. More detailed interpretation can be found from OSU Extension Fact PSS-2903 Nitrate Toxicity in Livestock. The most reliable way to find out nitrate in the hay is to collect a representative sample and have it tested by a laboratory. OSU Extension Fact PSS-2589 Collecting Forage Samples for Analysis highlights the proper techniques to collect forage samples. Samples can be submitted for nitrate and other forage quality analyses to the Soil, Water and Forage Analytical Laboratory in Stillwater through the local county extension office. We normally have the results ready within 24 hours form the time when sample is received by the lab. However, many samples we receive at the lab were not sampled properly. More attention should be paid on sampling standing forage, such as a haygrazer by following the right procedures:
Clip at least 20 representative plants at grazing or harvesting height from the suspected area. Cut the whole plants (include leaves and heads) into 2-3” long pieces, combine and mix well in a bucket.
Fill the cut sample into a forage bag. Use quartering to reduce the amount if there is too much sample to send to a lab.
Put the forage bag into a plastic bag will give you more accurate moisture content, but never put plastic bags inside our forage bags.
There is also a quick screening test using diphenylamine at your county extension office. This video shows how to properly use the test kit: https://www.youtube.com/watch?v=vArUP6KFQFI&feature=youtu.be
Hailin Zhang
Department of Plant and Soil Sciences