Laboratory evaluation of Liquid Calcium
Liquid calcium products have been around for a long time. The vast majority of these products are either a calcium chloride or chelated calcium base which is now commonly found with the addition of a humic acid, microbial, or micronutrient. Many of these make promises such as “raises your soil pH with natural, regenerative, liquid calcium fertilizers that correct soil pH quickly, efficiently, and affordably!”. From a soil chemistry aspect the promise of adding 3 to 5 gallons of a Ca solution, which is approximately 10% Ca, will raise the soil pH is impossible on a mass balance approach. In this I mean that to increase the pH of an acid soil {soil pH is the ratio of hydrogen (H) and hydroxide (OH) in the soil, and having an acid soil means the concentration of H is greater than that of OH} requires a significant portion of the H+ that is in solution and on soil particle to be converted to OH, or removed from the system entirely.
The blog below walks through the full chemical process of liming a soil but in essence to reduce the H+ concentration we add a cation (positively charged ion) such as Ca or magnesium (Mg) which will kick the H+ of the soil particle and a oxygen (O) donator such as CO2 with ag lime or (OH)2 which is in hydrated lime. Each of these O’s will react with two H’s to make water. And with that the pH increases.
However regardless of the chemistry, there is always a lot of discussion around the use of liquid calcium Therefore we decided to dig into the question with both field and laboratory testing. This blog will walk through the lab portion.
This was a laboratory incubation study. The objective was to evaluation the impact of the liquid Ca product (LiqCa**) on the soil pH, buffer capacity, Ca content and CEC of two acidic soils. LiqCa was applied at three rates to 500 g of soil. The three rates were equivalent to 2, 4, and 6 gallon per acre applied on a 6” acre furrow slice of soil. One none treated check and two comparative products were also applied. HydrateLime (CaO) as applied at rate of Ca equivalent to the amount of Ca applied via LiqCa, which was approximately 1.19 pounds of Ca per acre. Also AgLime (CaCO3) was applied at rates equivalent to 1, 2, and 4 ton effective calcium carbonate equivalency (ECCE). The Ag lime used in the study had a measured ECCE of 92%. The two soils selected for both acidic but had differing soil textures and buffering capacities. The first LCB, had an initial soil pH (1:1 H2O) of 5.3 and a texture of silty clay loam and Perkins had a initial pH of 5.8 and is a sandy loam texture. Both soils had been previously collected, dried, ground, and homogenized. In total 10 treatments were tested across two soils with four replications per treatment and soil.
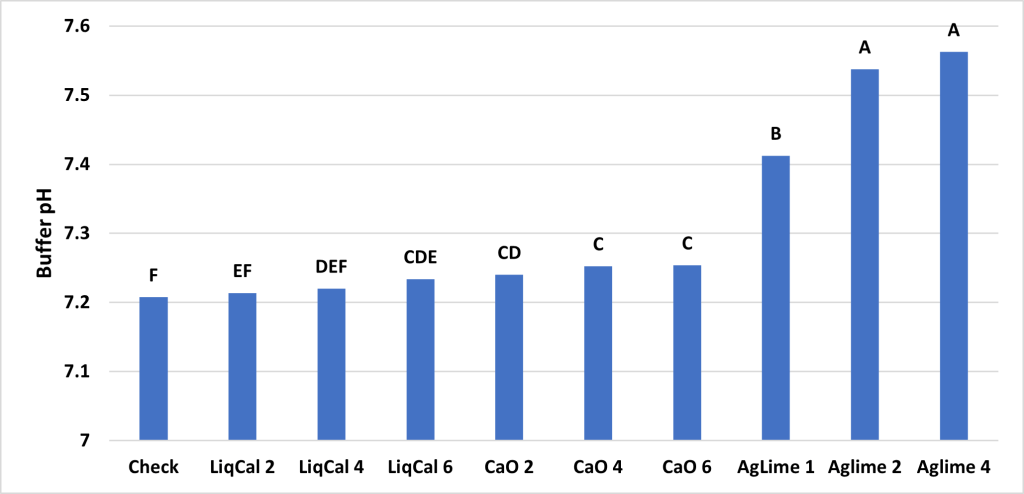
Project protocol, which has been used to determined site specific liming and acidification rates, was to apply the treatments to 500 grams of soil. Then for a period of eight weeks this soil wetted and mixed to a point of 50% field capacity once a week then allowed to airdry and be mixed again. At the initiation and every two weeks after soil pH was recorded from each treatment. The expectation is that soil pH levels will change as the liming products are impacting the system and at some point, the pH reaches equilibrium and no longer changes. In this soil that point was week six however the trail was continued to week eight for confirmation. See Figures 1 and 2.


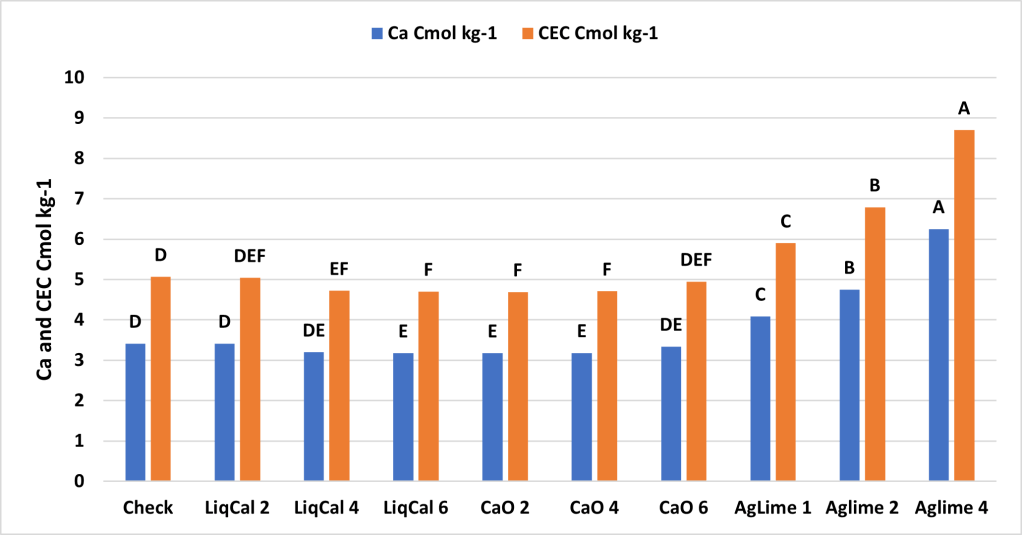
ANOVA Main effect analysis showed that Soil was not a significant effect so therefore both soils were combined for further analysis. Figure 3 shows the final soil pH of the treatments with letters above bars representing significance between treatments. In this study all treatments were significantly greater than the check with exception of LiqCal 2 and CaO 6. Neither LiqCal or CaO treatments reached the pH level of Aglime, regardless of rate.

Summary
The incubation study showed that application of LiqCal at a rate of 4 and 6 gallons per acre did significantly increase the soil pH by 0.1 pH units and 6 gallons per acre increased the Buffer index above the check by 0.03 units. Showing the application of LiqCal did impact the soil. However the application of 1 ton of Ag lime resulted in significantly great increase in soil pH, 1.0 units by 8 weeks and a buffer index change of 0.2 units. The Aglime 1 was statistically greatly than all LiqCal treatments. Ag lime 2 and 4 were both statistically greater than Ag lime 1 with increasing N rate with increasing lime rate. Given the active ingredient listed in LiqCal is CaCl, this result is not unexpected. Ag lime changes pH by the function of CO3 reacting H+ in large quantities. In a unsupported effort a titration was performed on LiqCal, which show the solution was buffered against pH change. However it was estimated that a application of approximately 500 gallons per acre would be needed to sufficiently change the soil pH within a 0-6” zone of soil.
Results of the field study.
https://osunpk.com/2025/06/02/field-evaluation-of-lime-and-calcium-sources-impact-on-acidity/
Take Home
The application of a liquid calcium will add both calcium and chloride which are plant essential nutrients and can be deficient. In a soil or environment suffering from Cl deficiency specifically I would expect an agronomic response. However this study suggest there is no benefit to soil acidity or CEC with the application rates utilized (2, 4, and 6 gallon per acre).
** LiqCal The product evaluated was derived from calcium chloride. It should be noted that since the completion of the study this specific product used has changed its formulation to a calcium chelate. This change however would not be expected to change the results as the experiment did include a equivalent calcium rate of calcium oxide.
Other articles of Interest
https://extension.psu.edu/beware-of-liquid-calcium-products
https://foragefax.tamu.edu/liquid-calcium-a-substitute-for-what/
Any questions or comments feel free to contact me. b.arnall@okstate.edu
Field evaluation of lime and calcium sources impact on Acidity.
At the same time we initiated a lab study looking at the application of LiqCal https://osunpk.com/?p=2096 , we also initiated a field trial to look at the multi-year application of LiqCal, Pelletized Lime and Ag-Lime.
A field study was implemented on a bermudagrass hay meadow near Stillwater in the summer of 2019. The study looked to evaluate the impact of multiple liming / calcium sources impact on forage yield and soil properties. This report will focus on the impact of treatments on soil properties while a later report will discuss the forage results.
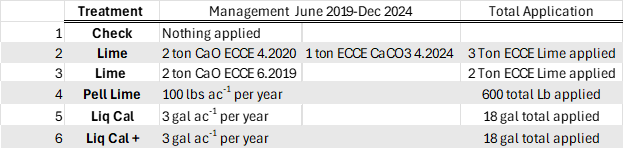
Table 1. has the management of the six treatments we evaluated, all plots had 30 gallons of 28-0-0 streamed on each spring in May. Treatment 1 was the un-treated check. Treatment 2, was meant to be a 2 ton ECCE (Effective Calcium Carbonate Equivalency) Ag Lime application when we first implemented the plots in 2019, but we could not source any in time so we applied 2.0 ton ECCE hydrated lime (CaO) the next spring. The spring 2023 soil samples showed the pH to have fallen below 5.8 so and Ag lime was sourced from a local quarry and 1.0 ton ECCE was applied May 2024. Treatment 3, was meant to complement Treatment 2 as an additional lime source of hydrated lime, it was applied June 2019. My project has used hydrated lime as a source for many years as it is fast acting and works great for research. Treatment 4 had 100 lbs. of pelletized lime applied each spring. The 100 lbs. rate was based upon recommendation from a local group that sells Pell lime. Treatments 5 and 6 were two liquid calcium products *Liq Cal * and **Lig Cal+ from the same company. The difference based upon information shared by the company was the addition of humic acid in the Liq Cal+ product. Both LiqCal and LiqCal+ where applied at a rate of 3 gallons per acre per year, with 17 gallons per acre of water as a carrier. Table 1, also shows total application over the six years of the study.
After six years of applications and harvest it was decided to terminate the study. The forage results were intriguing however little differences where seen in total harvest over the six years, highlighting a scenario I have encountered in the past on older stands of bermuda. That data will be shared in a separate blog.
The soils data however showed exceptionally consistent results.
In February of 2025 soil samples were collected from each plot at depths of 0-3 inch’s and 0-6 inches (Table 2.). It was our interest to see if the soil was being impacted below the zone we would expect lime and calcium to move without tillage, which if 0-3″. Figure 1. below shows the soil pH of the treatments at each depth. In the surface (blue) the Ag Lime and Hydrated lime treatments both significantly increased from 4.78 to 6.13 and 5.7 respectively. While the Pel lime, LiqCal and LiqCal+ had statistically similar pH’s as the check at 4.8, 4.65, and 4.65. It is important to note that the Ag Lime applied in May of 2024 resulted in a significant increase in pH from the 2019 application of Treatment 3. The Spring of 2024 soil samples showed that the two treatments ( 2 and 3 ) were equivalent. So within one year of application the Ag lime significantly raised soil pH.
As expected the impact on the 3-6″ soil pH was less than the surface. However, the Ag Lime and Hydrated lime treatments significantly increased the pH by approximately 0.50 pH units. This is important data as the majority of the literature suggestion limited impact of lime on the soil below the 3″ depth.

The buffer pH of a soil is used to determine the amount of lime needed to change the soils pH. In Figure 2. while numeric differences can be seen, no treatment statistically impacted the buffer pH at any soil depth.

The soil calcium level was also measured. As with 0-3″ pH and Buffer pH the Ag Lime and Hydrated lime had the greatest change from the check. These treatments were not statistically greater than the Pell Lime but where higher than the LiqCal and LiqCal+.
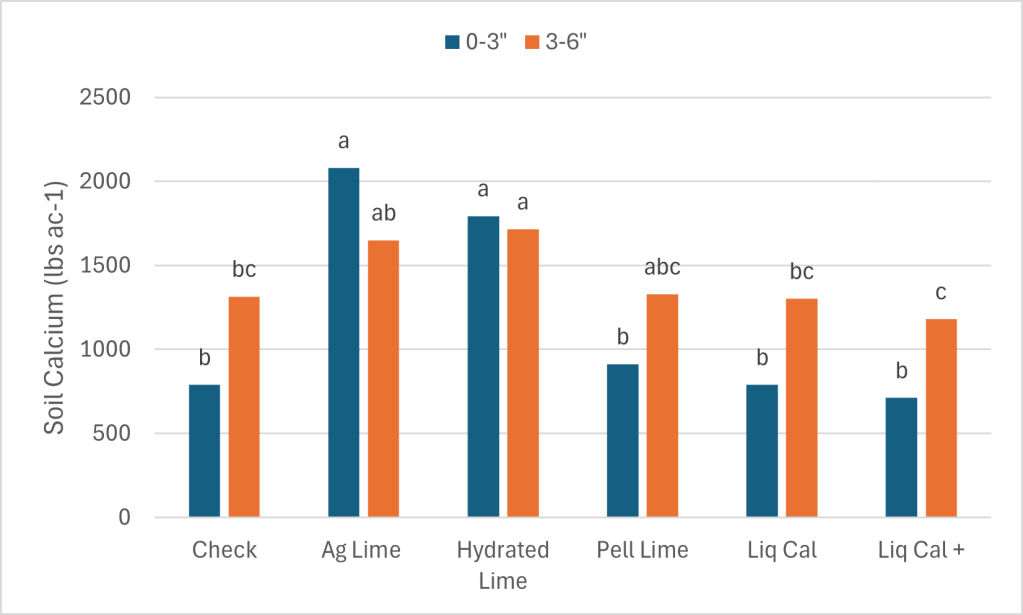

Each value is the average of four replicates.
Take Homes
In terms of changing the soils pH or calcium concentration, as explained in the blog https://osunpk.com/2023/01/24/mechanics-of-soil-fertility-the-hows-and-whys-of-the-things/, it takes a significant addition of cations and oxygens to have an impact. This data shows that after six years of continued application of pelletized lime and two liquid calcium products the soil pH did not change. While the application of 2 ton ECCE hydrate lime did.
Also within one year of application Ag lime the soil pH significantly increased.
* LiqCal The product evaluated was derived from calcium chloride. It should be noted that since the completion of the study this specific product used has changed its formulation to a calcium chelate. This change however would not be expected to change the results as the experiment did include a equivalent calcium rate of calcium oxide.
** LiqCal+ The product evaluated was derived from calcium chloride. It should be noted that since the completion of the study this specific product used has changed its formulation. The base was changed from calcium chloride to a calcium chelate. Neither existing label showed Humic Acid as a additive, however the new label has a a list of nutrients at or below 0.02% (Mg, Zn, S, Mn, Cu, B, Fe) and Na at .032% and is advertised as having microbial enhancements.
Any questions or comments feel free to contact me. b.arnall@okstate.edu
Sorghum Nitrogen Timing
Contributors:
Josh Lofton, Cropping Systems Specialist
Brian Arnall, Precision Nutrient Specialist
This blog will bring in a three recent sorghum projects which will tie directly into past work highlighted the blogs https://osunpk.com/2022/04/07/can-grain-sorghum-wait-on-nitrogen-one-more-year-of-data/ and https://osunpk.com/2022/04/08/in-season-n-application-methods-for-sorghum/
Sorghum N management can be challenging. This is especially true as growers evaluate the input cost and associated return on investment expected for every input. Recent work at Oklahoma State University has highlighted that N applications in grain sorghum can be delayed by up to 30 days following emergence without significant yield declines. While this information is highly valuable, trials can only be run on certain environmental conditions. Changes in these conditions could alter the results enough to impact the effect delay N could have on the crop. Therefore, evaluating the physiological and phenotypic response of these delayed applications, especially with varied other agronomic management would be warranted.
One of the biggest agronomic management sorghum growers face yearly is planting rate. Growers typically increase the seeding rate in systems where specific resources, especially water, will not limit yield. At the same time, dryland growers across Oklahoma often decrease seeding rates by a large margin if adverse conditions are expected. If seeding rates are lowered in these conditions and resources are plentiful, sorghum often will develop tillers to overcome lower populations. However, if N is delayed, there is a potential that not enough resources will be available to develop these tillers, which could decrease yields.
A recent set of trials, summarized below, shows that as N is delayed, the number of tillers significantly decreases over time. Furthermore, the plant cannot overcompensate for the lower number of productive heads with significantly greater head size or grain weight.
This information shows that delaying sorghum N applications can still be a viable strategy as growers evaluate their crop’s potential and possible returns. However, delayed N applications will often result in a lower number of tillers without compensating with increased primary head size or grain weight.



This date on yield components is really interesting when you then consider the grain yield data. The study, which is where the above yield component data came from, was looking at population by N timing. The Cropping Systems team planted 60K seeds per acre and hand thinned the stands down to 28 K (low) and 36K (high). The N was applied at planting, 21 days after emergence, and 42 days after emergence. The rate of N applied was 75 lbs N ac. It should be noted both locations were responsive to N fertilizer.

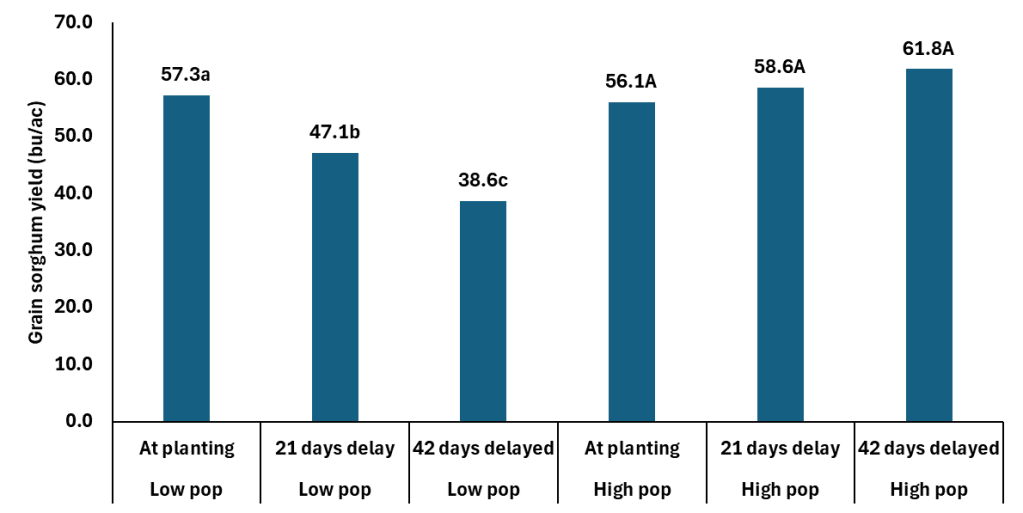
In the data you can without question see how the delayed N management is not a tool for any of members of the Low Pop Mafia. However those at what is closer to mid 30K+ there is no yield penalty and maybe a yield boost with delayed N. The extra yield is coming from the slightly heavier berries and getting more berries per head. Which is similar to what we are seeing in winter wheat. Delaying N in wheat is resulting in fewer tillers at harvest, but more berries per head with slightly heavier berries.
Now we can throw even more data into the pot from the Precision Nutrient Management Teams 2024 trials. The first trial below is a rate, time and source project where the primary source was urea applied in front of the planter for pre in range of rates from 0-180 in 30 lbs increments. Also applied pre was 90 lbs N as Super U. Then at 30 days after planted we applied 90 lbs N as urea, SuperU, UAN, and UAN + Anvol.
Pre-plant urea topped out at 150 lbs of Pre-plant (57 bushel), but it was statistically equal to 90 lbs N 51 bushel. The use of SuperU pre did not statistically increase yield but hit 56 bushel. The in-season shots of 90 lbs of UAN, statistically outperformed 90 pre and hit our highest yeilds of 63 and 62 bushel per acre. The dry sources in-season either equaled their in preplant counter parts.

The Burn Study at Perkins, showed that the N could be applied in-season through a range of methods, and still result good yields. In this study 90 lbs of N was used and applied in a range of methods. The treatments for this study was applied on a different day than the N source. Which you can see in this case the dry untreated urea did quite well when when applied over the top of sorghum. In this case we are able to get a rain in just two days. So we did get good tissue burn but quick incorporation with limited volatilization.

Take Home:
Unless working in low population scenarios. The data show that we should not be getting into any rush with sorghum and can wait until we know we have a good stand. We also have several options in terms of nitrogen sources and method of application.
Any questions or comments feel free to contact Dr. Lofton or myself
josh.lofton@okstate.edu
b.arnall@okstate.edu
Funding Provided by The Oklahoma Fertilizer Checkoff, The Oklahoma Sorghum Commission, and the National Sorghum Growers.
Boosting Wheat Grain Protein: Smart Spray Strategies for Better Grain Quality
Brian Arnall, Precision Nutrient Management Specialist
Samson Abiola, PNM Ph.D. Student.
Wheat Protein and Technology challenge
For wheat growers, achieving both high yields and good protein content is a constant challenge. Wheat contributes about 20% of the world’s calories, making it a vital crop for global nutrition. Every season, we face the question of how to boost grain protein concentration (GPC) without sacrificing the yield.
Traditional approaches often involve applying more nitrogen (N) early in the season. While this can help, it is often wasteful, environmentally problematic, and does not always translate to higher protein levels at harvest. The effectiveness of N applications depends not just on timing but also on the spray technology used, including the N source, nozzle type, and droplet size. While protein premiums are never guaranteed, we wanted to develop recommendations prior to the need.
The Research Approach: Timing and Technology
Our research team conducted a comprehensive three-year study (2019-2022) across three Oklahoma locations (Perkins, Lake Carl Blackwell, and Chickasha) to investigate how different combinations of N sources, nozzle types, and droplet sizes affect protein when applied during flowering. We considered two N sources (urea-ammonium nitrate (UAN) and aqueous urea [Aq. Urea]). We also evaluated three nozzle types: Standard flat fan (FF) nozzles with a traditional 110° spray angle, 3D nozzles with three-dimensional spray patterns that enhance canopy penetration, Twin (TW) nozzles with dual forward and rear facing sprays (30° forward and backward)
Finally, we tested both fine droplets (below 141 microns) and coarse droplets (≥141 microns). All applications were made at flowering i.e., when you start seeing yellow anthers sticking out of the wheat heads. Both UAN and Aq. urea were applied at a 20 gpa application rate with a 1:1 dilution with water delivering approximately 30 lbs. of N per acre.
What We Found: More Protein Without Hurting Yield
The big news? Spraying N at flowering boosted wheat protein by 12% without sacrificing yield. This held true across fields yielding anywhere from 30 to 86 bushels per acre. Why doesn’t it hurt yield? By flowering time, your wheat has already “decided” how many heads and kernels it will produce. The N you spray at this stage goes straight to building protein in those existing kernels.
One important caution: Mother Nature still calls the shots, so keep an eye on the forecast before planning your application. If the weather is hot and dry, this is not a good idea. First, those environments typically result in higher protein anyways. But low humidity will significantly increase the likelihood of burn.
Lake Carl Blackwell Findings: UAN Takes the Lead
At our Lake Carl Blackwell site, we saw our highest protein levels reaching up to 16.3% in some plots. In 2020-21, UAN clearly beat Aq. urea (14.7% vs. 14.0% protein). Both were much better than not applying any N at flowering (13.1%) (Figure 1A). Also, the 3D nozzle gave us the highest protein (14.7%), outperforming the control but performing similarly to FF (14.0%) and TW nozzles (14.2% (Figure 1B). The next year (2021-22) showed us something interesting, the combination of N source and droplet size really matters. UAN with fine droplets hit 14.6% protein, similar to UAN with coarse droplets (14.4%) and Aq. urea with coarse droplets (14.3%), but Aq. urea with fine droplets fell behind at just 13.8% (Figure 1C).

Chickasha Results: Matching Your N to the Right Droplet Size
At Chickasha, protein ranged from 10.1% to 13.8% across the two years we studied. In 2021-22, UAN beat Aq. urea (12.7% vs. 12.2%), and both beat the control (11.8%) (Figure 2A). Also, the 3D nozzle (12.8%) outperformed both FF and TW nozzles (both 12.2%) (Figure 2B).
In 2020-21, we found that the combination of N source, nozzle type, and droplet size all worked together to affect protein. The winning combination was UAN with 3D nozzle and fine droplets (13.23% protein), which performed similarly to Aq. urea with TW nozzle and coarse droplets (13.18%) (Figure 2C). The least performer was Aq. urea with TW nozzles and fine droplets (12.20%) among the treatments. This shows how weather and growing conditions can change which factors matter most from year to year

Perkins Results: Getting Every Detail Right
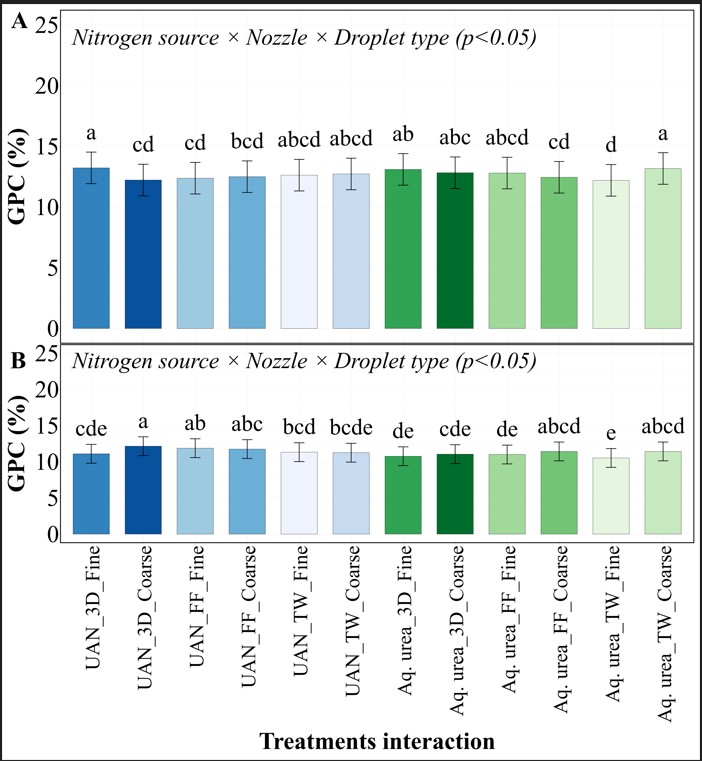
At our Perkins site, we saw protein levels ranging from 10% to 13.1%. Here, the combination of all three factors (N source, nozzle type, and droplet size) made a huge difference. The best setup was UAN with 3D nozzle and coarse droplets (12.2% protein). The worst was Aq. urea with TW nozzle and fine droplets (10.5%) (Figure 3). That’s a 15% difference that could mean the difference between premium and feed-grade wheat!
UAN consistently outperformed Aq. Urea across all setups. For example, UAN with 3D nozzle and coarse droplets produced 10% higher protein than the same setup with Aq. Urea.
Equipment and Application Recommendations
Over the three years UAN consistently outperformed perform Aq. urea, showing there is no need for a special formulation and that commercially available UAN is all we really need as a source. While no nozzle type significantly stood out across all sites the 3-D nozzle did show up a couple times as being statistically better. So the important message would be that while the high tech nozzles could provide some value the traditional flat fan performed quite well. While some differences were seen in droplet size, the lack of consistency leads us to say focus on good coverage with limited drift.
Take-Home Messages
- Foliar N at flowering boosted wheat protein by 12% without affecting yield multiple growing seasons and locations. This increase was from 0.5 to nearly 2.0 % protein.
- Nitrogen source matters – UAN consistently outperformed Aq. Urea.
- Your spray technology mattered but not lot – and 3D nozzles generally gave the best results. The good ole flat fans nozzles still did quite will.
- Match droplet size to your setup – generally fine for UAN and coarse for Aq. Urea.
- This targeted approach enhances grain quality without sacrificing yield, potentially improving grain prices and profitability while using N more efficiently.
- Mother Nature still calls the shots, so keep an eye on the forecast before planning your application. If the weather is hot and dry, this is not a good idea. First, those environments typically result in higher protein anyways. But low humidity will significantly increase the likelihood of burn.
This blog was written based upon the data published in the manuscript “Optimizing Spray Technology and Nitrogen Sources for Wheat Grain Protein Enhancement” which is available for free reading and downloading at https://www.mdpi.com/2077-0472/15/8/812
Any questions or comments feel free to contact me. b.arnall@okstate.edu
Nitrogen and Sulfur in Wheat
Brian Arnall, Precision Nutrient Management Specialist
Samson Abiola, PNM Ph.D. Student.
Nitrogen timing in wheat production is not a new topic on this blog, in-fact its the majority. But not often do we dive into the application of sulfur. And as it is top-dressing season I thought it would be a great opportunity to look at summary of a project I have been running since the fall of 2017 which the team has call the Protein Progression Study. The objective was to evaluate the impact of N and S application timings on winter wheat grain yield and protein. With a goal of looking at the ratio of the N split along with the addition of S and late season N and S, in such a way that we could determine BMP for maximizing grain yield and protein.

My work in the past has shown two things consistently, that spring N is better on the average and S responses have been limited to deep sandy soils in wet years. Way back when (2013) on farm response strips showed high residual N at depth and no response to S. https://osunpk.com/2013/06/28/response-to-npks-strips-across-oklahoma/. But there has been a lot of grain grown since that time expectations are that we should/are seeing an increase in S response. In fact Kansas State is seeing more S response, especially in the well drained soils in east half of the state.
Some KSU Sulfur works.
https://www.ksre.k-state.edu/news/stories/2022/04/video-sulfur-deficiency-in-wheat.html
https://eupdate.agronomy.ksu.edu/article/sulfur-deficiency-in-wheat-364-1
Click to access sulphur-in-kansas-plant-soil-and-fertilizer-considerations_MF2264.pdf
So the Protein Progression Project was established in 2017 and where ever we had space we would drop in the study. So in the end across six seasons we had 13 trials spread over five locations. Site-years varied by location: Chickasha (2018-2022), Lake Carl Blackwell (2018-2023), Ballagh (2020), Perkins (2021), and Caldwell (2021).

First lets just dive into the the N application were we looked at 100% pre vs 50-50 split and 25-75 split (Table 2.) Based upon the wealth of previous work https://osunpk.com/2022/08/26/impact-of-nitrogen-timing-2021-22-version/, its not much of a surprise that split application out preformed preplant and that having the majority applied in-season tended to better grain yields and protein values.

This next table is were things get to be un-expected. While the data below is presented by location, we did run each site year by itself. In no one site year did S statistically, or numerically increase yield. As you can see in Table 2 below, the only statistical response was a negative yield response to S. And you can not ignore the trend that numerically, adding S had consistently lower yields. Even more surprising was the same trend was seen in Protein.
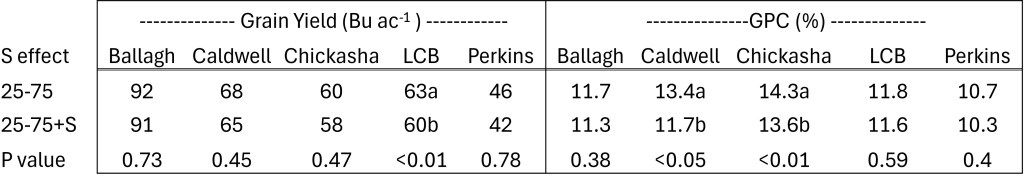
One aspect of Protein Progression trials were that while 0-6″ soil test S tended to be low. We would often find pretty high levels of S when we sampled deeper, especially when there was a clay increase with depth. Sulfur tends to be held by the clay in our subsoil. We are also looking at better understanding the relationship between N and S. In fact a review article published in 2010 discussed that the N and S ratio can negative influence crop production when either one of the elements becomes un-balanced. For example we are seeing more often in corn that when N is over applied we can experience yield loss, unless we apply S. Meaning at 200 lbs of N we make 275 BPA, at 300 N lbs we make 250, but 300 N plus 20 S we can make 275 again. Part of the rationale is that excessive N limits S mineralization. On the flip side if S is applied while N is deficient and yield decrease could be experienced. Maybe that is what we are seeing in this date. Either way, this data is why the Precision Nutrient Management program is spending a fair amount of efforts in understanding the N x S relationship in wheat (which we are looking at milling quality also) and corn.
A quick dive into increasing protein with late N applications. At three of the five location GPC was significantly increased with Late N. In most cases the anthesis (flowering) application was the highest with exception of Caldwell. We will have another blog coming out in a month that digs into anthesis applied N at a much deeper level, looking at source, nozzle and droplet sizes.

Looking at this study in a vacuum we can say that it probably best to split apply your N and that in central and northern Ok the addition of S in rainfed wheat doesn’t offer great ROI. If I look at the whole picture of all my work and experience I would offer this. For grain only wheat, the majority if not all N should be applied in-season sometime between green up and two weeks after hollow stem. I have had positive yield responses to S applied top-dress, but it has always been deep sandy soils and wet seasons. I have not have much is any response to S in heavier soil, especially if there is a clay increase in the two feet of profile. So my general S recommendation is 10 lbs in sandy soils and if you show low soil test S in heavier ground and you are trying to push grain yields, then you could consider the addition of S as a potential insurance. That said, I haven’t seen much proof of it.
Take Homes
* Split application of nitrogen resulted in higher grain yields and protein concentrations when compared to 100% preplant.
* Putting on 75% of the total N in-season tended to result in higher grain yields and protein concentrations when compared to 50-50 split.
* Adding 10 lbs of S topdress did not result in any increase in grain yield or protein.
A big Thanks to the collaborators providing on-farm locations for this project. Ballagh Family Farms, Turek Family Farms and Tyler Knight.
Citation. Jamal, A.,*, Y. Moon, M. Abdin. 2010 Review article. Sulphur -a general overview and interaction with nitrogen. AJCS 4(7):523-529 (2010). ISSN:1835-2707.
Any questions or comments feel free to contact me. b.arnall@okstate.edu
Management of soybean inoculum
Josh Lofton, Cropping Systems Specialist
Brian Arnall, Precision Nutrient Management Specialist
Soybean, a legume, can form a symbiotic relationship with Bradyrhizobium japonicum (Kirchner, Buchanan) and create their N to supplement crop demands. However, this relationship depends upon these beneficial microorganisms’ presence and persistence in the soil. This specific strain of microorganisms is not native to Oklahoma and thus must be supplemented using inoculum as a seed treatment. However, the use of inoculums alone does not guarantee a successful relationship. Handling, storage, soil conditions, and other factors can impact the ability of these microorganisms to do their job.
Soybean nitrogen demand is high, with most reports indicating that soybeans need 4.5 to 5.0 pounds of nitrogen per bushel of grain yield. This means that a 30-bushel crop requires between 135 and 150 pounds of nitrogen per acre (in comparison, corn and wheat need only 0.8 or1.6 pounds, respectively). This relationship has been shown to supply an equivalent of 89 lbs of N to the soil. In the previous example, these bacteria could fulfill 50-90% of nitrogen demand, reducing input costs significantly.
However, the bacteria associated with soybean inoculum are living organisms. Therefore, the conditions they experience before being applied to the seed and after treatment (including both before and following planting) can significantly impact their relationship with the soybean plant and, thus, their ability to provide N to the plant. By introducing a high concentration of bacteria near the seed and emerging root, this symbiotic relationship is more likely to be established quickly.
The importance of using inoculum is often debated in Oklahoma, particularly given the fluctuating prices of commodities and inputs. A recent assessment of various soybean-producing areas throughout the state revealed that most fields experienced advantages from incorporating soybean inoculation (Figure 1).

These benefits can be seen when the inoculum maintains viability until it is planted. It is always recommended that the bacteria be stored in a cool, dark environment before application on the seed. These conditions help preserve the survival of these bacteria outside of the host relationship. An evaluation of soybean inoculant after being stored short-term in different conditions found that in as little as 14 days, viability can decrease when kept in non-climate-controlled conditions (Figure 2). Additionally, viability was further reduced at 21 days when stored at room temperature compared to a refrigerated system

However, conditions colder than this, such as the use of a freezer, can compromise survival as well. Storing inoculum in the freezer forms ice crystals within the living cells and damages the cell membranes, making the microorganisms less likely to be alive upon rethawing. Additional chemicals can be added to increase the viability of long-term storage and sub-freezing temperatures. From an application standpoint, a new product should be purchased if additional storage is needed beyond short-term storage.
An additional question frequently arises: “How often should I inoculate my soybean?” As mentioned, these bacteria are not native to Oklahoma. As a result, they are not well adapted to survive in our environment and must outcompete native populations in the soil. Additionally, periods of hot and dry conditions appear to reduce the bacteria’s ability to survive without a host, the soybean roots. These are conditions we often observe in Oklahoma systems. Therefore, inoculation should be applied with every soybean planting to ensure a sufficient population of these bacteria. These bacteria promote root nodulation and nitrogen fixation in the soil.
Other soil conditions, such as excessively dry or wet soils, high or low pH, and residual nutrients, can also impact the persistence of these microorganisms. Of these, soil pH has the biggest impact on the survival of these bacteria. High pH is less of a concern to Oklahoma production systems; however, soil with lower pH should be remediated. Like many bacterial systems, these bacteria optimally function at a pH range that closely resembles the ideal pH range for most crops. Lowering the soil pH below a critical threshold reduces the viability of the bacteria, hampers N-fixation processes, and diminishes the capacity of both the bacteria and soybean plants to form and maintain this relationship. While applying inoculum to soybean seeds in these adverse soil conditions can provide some advantages (Figure 3), but it often doesn’t increase yields. Therefore, inoculation with corresponding adjustments to soil pH represents the best approach.


While using inoculum is not a new concept, it is important to highlight the benefits it can provide when utilized correctly. The potential to reduce N input costs is attractive, but the effectiveness depends on proper handling, storage, and soil conditions until it can intercept the host. To maximize benefits, inoculum should be stored in a cool, dark environment and utilized in a timely manner. If there is doubt that there are not enough bacteria, an inoculum should be added. Oklahoma’s climate, particularly hot and dry conditions, can limit bacteria survival, reinforcing the need to treat the inoculum until it is in the ground carefully. Additionally, considering the soil environment is important to sustain the population of bacteria until it can inoculate its host. Emphasis on these small details can have a large impact on the plant’s ability to fix nitrogen and optimize productivity throughout the growing season.
TAKE HOMES
* Soybean requires more lbs of N per bushel than most grain crops.
* Soybeans symbiotic relationship with rhizobia can provide the majority of this nitrogen.
* Soybean rhizobia is not native to Oklahoma soils so should be added to first year soybean fields.
* Inoculum should be treated with care to insure proper nodulation.
* Due to Oklahoma’s climate and existing soil conditions rhizobia may not persist from year to year.
Any questions or comments feel free to contact Dr. Lofton or myself
josh.lofton@okstate.edu
b.arnall@okstate.edu
Appreciation of the Oklahoma Soybean Board for their support of this project.
A comparison of four nitrogen sources in No-till Wheat.
Jolee Derrick, Precision Nutrient Management Masters Student.
Brian Arnall, Precision Nutrient Management Specialist.
Nitrogen (N) fertilizer’s ability to be utilized by a production system is reliant upon the surrounding environment. The state of Oklahoma’s diverse climate presents unique challenges for producers aiming to apply fertilizers effectively and mitigate the adverse effects of unfavorable conditions on nitrogen fertilizers. To lessen the effect that unfavorable environments can have on N fertilizers, chemical additions have been introduced to base fertilizers to give the best possible chance at an impact. With that in mind, a study was conducted to investigate the impact of N sources and application timings on winter wheat grain yield and protein, aiming to identify both the agronomic effects of these sources and how variations in their timing may influence the crop. Included below is a figure of where and when the trials were conducted.

In each of the trials, four N sources (Urea, SuperU, UAN, and UAN + Anvol) were analyzed across a range of timings. The sources were categorized on two criteria: application type, distinguishing between dry and liquid sources, and the presence of additives versus non-additives. The two N sources were Urea and UAN. The other products in this study were SuperU and Anvol. SuperU is a N product that has Dicyandiamide (DCD) and N- (n-butyl) thiophosphoric triamide (NBPT) incorporated into a Urea base. Anvol is an additive product which contains NBPT and Duromide and can be incorporated with dry or liquid N sources.
For additional clarification, N- (n-butyl) thiophosphoric triamide is a urease inhibitor which prevents the conversion of urea to ammonia. Duromide is a molecule which is intended to slow the breakdown of NBPT. DCD is a nitrification inhibitor that slows the conversion of ammonium to nitrate.
Urea is a stable molecule which in the presence of moisture is quickly converted to stable ammonium (NH4), however it can be converted to ammonia gas (NH3) by the enzyme urease beforehand. Additionally, when urea is left on the soil surface and not incorporated via tillage or ½ inch of a precipitation event, the NH4 that was created from urea can be converted back to NH3 and gasses off. So, the use of urease inhibitors is implemented to allow more time for incorporation of the urea into the soil.
Ammonium in the soil is quickly converted to nitrate (NO3) by soil microbes when soil temperature is above 50F°. When N is in the NO3 form it is more susceptible to loss through leaching or denitrification. Therefore, nitrification inhibitors are applied to prevent the conversion of NH4 to NO3.
All treatments were applied at the same rate of 60 lbs of N ac-1, which is well below yield goal rate. A lower N rate was chosen to allow the efficacy of the products to express themselves more clearly, rather than a higher rate that may limit the ability to determine differences between product and rate applied. Furthermore, dry N sources were broadcasted by hand across the plots while liquid sources were applied by backpacking utilizing a handheld boom with streamer nozzles. Application timing dates were analyzed by identifying the growing degree days (GDD) associated with each timing which were correlated with the Feekes physiological growth chart displayed in Figure 3. Over the span of the study, N has been applied over six stages of growth. The range of application dates stems from the fact that it is difficult to get across all the ground exactly when you need to.


Over four years this study was replicated 11 times. Of those 11 site years, three did not show a response to N, so they were removed from further analysis. The graph above shows the average yield of each respective source (across all locations and timings). The data shows there is a statistical difference between SuperU and Urea vs. UAN, but no statistical difference between UAN treated Anvol and any other source. The data indicates that on average, a dry source resulted in a higher yield than when a liquid source was applied. This makes sense considering that in many cases, wheat was planted in heavy residue during cropping seasons that experienced prolonged drought conditions. Therefore, it is thought that a liquid source can get tied up in the residue. This was first reported in a previous blog posting, Its dry and nitrogen cost a lot, what now?, and years later, the same trends in new data indicate the same conclusion.
If we look at all timings and site years averaging together there is no statistical difference between a raw N source and its treated counterpart. This result is not surprising as we would expect that not all environments were conducive to loss pathways that the products prevented. Basically, we would not expect a return on investment in every single site year, and therefore you do not see broad sweeping recommendations. There was a 2-bushel difference between UAN and UAN Anvol. As this was a numerical difference, not a statistical one, I would say that while the yield advantage was not substantial there may be economic environments that would suggest general use.

While the evaluation of the four sources across all timings and locations showed some interesting results, this work was performed to see if there was a timing of application which would have a higher probability of a safened N returning better yields. As you look at the chart above it is good to remember the traditional trend for precipitation in Oklahoma, where we tend to start going dry in November and stay dry through mid-January. Rain fall probability and frequency starts to increase around mid-February, but moisture isn’t consistent until March. This project was performed during some of the dryest winters we have seen in Oklahoma. Also, just a note, since the graph above combines all the sites that have differing application dates the absolute yields are a bit deceiving. For example, the Nov and Feb timings include the locations with our highest yields 80+ bpa per acre, while January and February include our lowest. So, the way this data is represented we should not draw conclusions about best time for N app. For that go read the blogs Impact Nitrogen timing 2021-2022 Version and Is there still time for Nitrogen??


Figure 6a and b. A: The mesonet rainfall totals for Oct -Dec for the Lake Carl Blackwell research station for 2020-2023 A: The mesonet rainfall totals for Jan – April for the Lake Carl Blackwell research station for 2021-2024. The black line on both graphs is the 10 year average.
Now about the source by time. While it’s not always statistical you can see that the dry sources tend to outperform the liquid sources at most timings. Also, while there is never a statistical difference in the raw product and safened, there are trends. SuperU tended to have higher yields than urea when applied in Nov and Dec. It could be hypothesized that the addition of a nitrification inhibitor may have added value, however the UAN + Anvol in November also showed a positive response that would point to the value being derived from urease inhibition. As we move into the period of more consistent rainfall the differences between products start declining, which also makes sense.
The following figures illustrate rainfall events following N application, with the application dates indicated by arrows. Figure 7 corresponds to our trial conducted in 2020-2021, which revealed no statistical differences among the N sources for any timing. However you can see that for the first timing (orange bars) which received 0.7″ of rain two days after application that the yields are uniform than the grey bars with both safened products are numerically greater than the raw product, just makes since as a 0.1″ precipitation event happened 6 days application application and it wasn’t until day 9 that a good incorporating rainfall occurred.

Figure 8 has data the same location one year later, during which we observed statistically significant differences among the dry and liquid N sources. The environmental conditions during 2022 were drier, impacting the incorporation of N applications. The lack incorporating rainfall likely led to tie-up of the UAN on/in the residue, limiting access to plant available N.

It is essential to highlight the environmental conditions encountered throughout this project. From 2020 to 2023, moderate to extreme drought conditions were prevalent. During this period, the influence of a La Niña led to reduced moisture availability. For the first time in an extended period, the 2023-2024 wheat year began under a strong El Niño, which typically results in increased moisture accumulation compared to its La Niña counterpart. Currently, there are indications that a return to a La Niña system may occur by the end of the year, raising the expectation of a potential reversion to drought conditions. Consequently, this research may provide producers with options to consider regarding sources of N application in their production systems.
We plan to update this blog with a deeper analysis of the results seen at each location as soon as possible. But for now, we wanted to share the early look.
At this point the reason for the liquid yield gap is speculation. It could be increased loss via ammonia volatilization or it could be immobilization of the N by microbes. The next step of this process is to understand 1) is the UAN tied up in the residue via immobilization via microbes or is it lost to volatilization. 2) If tied up, what is the time frame between application and immobilization.
Take Home:
- It was observed that during low moisture conditions, dry N sources had significantly better results than liquid counterparts in no-till winter wheat production.
- On average, additive products had no significant impact on grain yield versus base fertilizers, however, when evaluated by location, differences could be found. Responses usually correlated with post-application weather conditions.
- When adequate precipitation was received shortly after application, N sources did not show differences.
Any questions or comments feel free to contact me. b.arnall@okstate.edu
In-Furrow Placement of Urea Products with Wheat Seed
Its that time of year I always get the question of “How much urea can I put in the furrow?”. My answer is always two fold first, I wouldn’t recommend it, its a risky venture. Even though I know some do it. Second, my research shows very little is any value from N in furrow. I like P but N just doesn’t show me any return. So for me the process is high risk, with little or no potential for return. But with blog I turn to our purple friend up north to share what their research has sown.
Brian
Guest Authors Kansas State University
Lucas Haag, Agronomist-in-Charge, Southwest Research-Extension Center, Tribune lhaag@ksu.edu
Alan Schlegel, retired
Dorivar Ruiz Diaz, Nutrient Management Specialist ruizdiaz@ksu.edu
To save time and cost, some wheat producers may be thinking about adding a little extra nitrogen (N) as urea or UAN to their phosphorus fertilizer through the drill with the seed. This would either be in addition to, or instead of, any preplant N applications.
While a minimum preplant N application of 20 to 40 lbs N per acre is often desirable, especially in no till production systems, there are risk involved when placing urea containing fertilizers in direct seed contact. Traditionally, we have suggested that no urea or UAN solution be placed in contact with the seed. With the continued adoption of air-seeders a common question we receive from producers is can urea, or enhanced urea products be placed in-furrow.
Methods of early-season nitrogen applications
If the starter fertilizer can’t be “spiked” with urea to add extra N, how can the necessary 20 to 40 pounds of N be applied? Subsurface banding (knifing) of N as either anhydrous ammonia, liquid UAN, or dry product will result in the greatest N use efficiency by the wheat crop. This is especially true for no-till wheat production.
If knifed N applications are not used, the next best application method would be surface banding (dribbling) of UAN solution in streams on 15- to 18-inch centers. Broadcasting urea, ammonium nitrate, or UAN applications are not generally as efficient as subsurface banding, but they are often the best choice due to equipment, logistics, or weed management considerations. Broadcast applications of N will have the most consistent performance if followed by light incorporation, precipitation, or irrigation.
Direct seed placement of nitrogen
When placing starter fertilizer in direct contact with wheat seed, producers should use the following guidelines:

The problem with placing urea-containing fertilizer with the seed is that urea is initially converted to ammonia and may be toxic to plant roots if the wheat seed is placed in direct contact with the fertilizer. Producers may hear of someone who has placed urea in direct seed contact and seemed to have no problems, but there are also many cases where urea-containing N fertilizers has injured the developing seedling and reduced or delayed emergence significantly. The risk of injury is greater in drier soils, at higher soil pH levels, and at higher N rates. High soil pH favors a higher concentration of ammonia as compared to ammonium as urea hydrolyzes. There is significant risk associated with placing urea-containing fertilizers in direct seed contact.
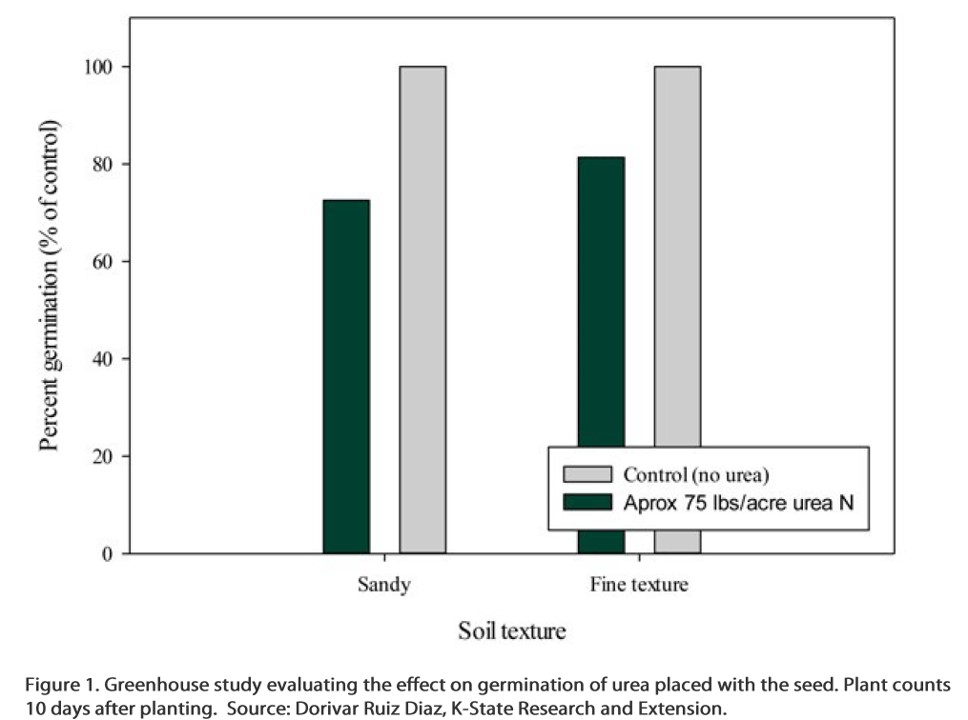
The chart below shows how soil texture affected the level of wheat germination when urea-N was applied with the seed in a K-State greenhouse study. The wheat was well watered in this study, but urea-N placed with the seed still reduced germination, especially in the sandy soil. The readings shown below were taken after 10 days. With the high rates of urea used in this study, it is possible that more damage to the seedlings would occur with time as the urea continues to hydrolyze into ammonia.
Field studies have also shown reduced wheat stands due to in-furrow placement of urea. Across 5 site years in western Kansas the placement of urea in-furrow has resulted in decreased stands at spring greenup compared to the control (Figure 2).

The stand reduction becomes especially noticeable at higher rate of N. One of the challenges of understanding the risk of seedling injury is that the magnitude of injury varies by field conditions an years. In some years very little reduction may be evident, even at higher rates of N, while in other years, stand reductions (and their associated impact on yield) is very evident. As an example at Tribune in 2017, reduction in stand caused by urea placement with seed, and their effect on yield were quite evident (Figures 3 and 4).
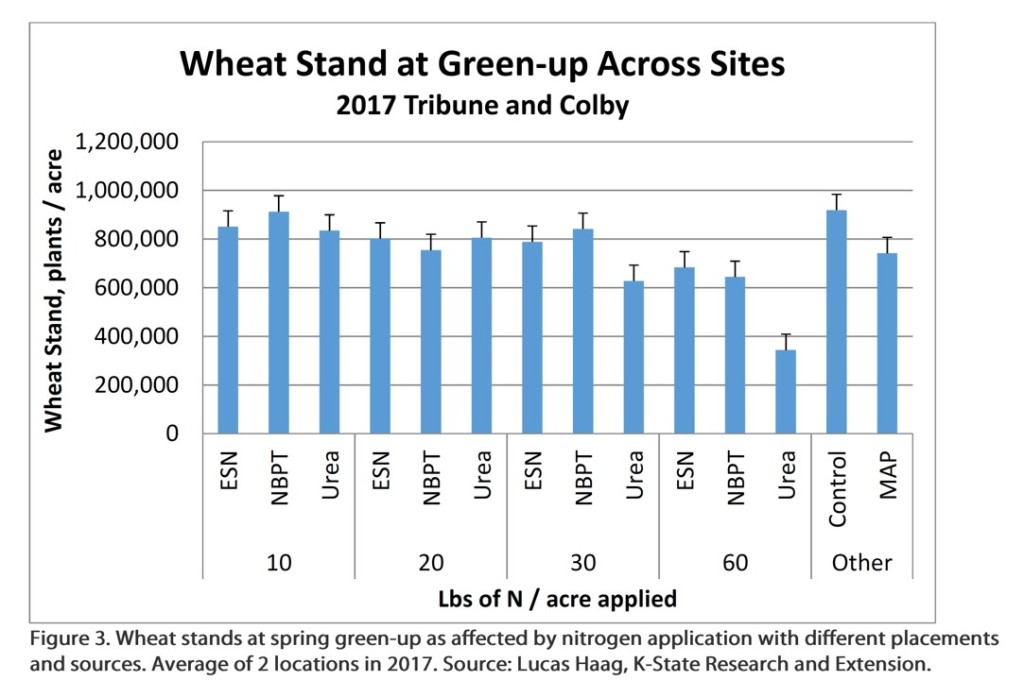

Stands were reduced 32 and 63% compared to the control when 30 and 60 lbs of N as urea were applied in-furrow (Figure 3). This resuled in yield reductions of 14 and 40%, respectively (Figure 4).
If you’d like to apply extra N directly in the seed furrow, one option is to use a controlled-release form of N, such as ESN. As shown in figure 4, at N application rates of 30 lbs/ac and less, where ESN-N was applied in-furrow, wheat yields were essentially the same as where the N was applied pre-plant, and higher compared to the same amount of N applied as urea. At the highest rate of application in the study, 60 lbs/ac, even ESN resulted in stand and grain yield reductions.
Also, air seeders that place the starter fertilizer and seed in a band an inch or two wide, or side band the fertilizer relative to the seed, provide some margin of safety because the concentration of the fertilizer and seed is lower in these diffuse bands. In this scenario, adding a little extra urea containing N fertilizers to the starter less likely to injure the seed – but it is still a risk.
Here is a great video by Dr. Haag.
How do you handle your soil sample….
It’s that time of year where wheat producers are in all stages of prep across the state, the graze out folks are wondering when the rains will hit to get the dusted in wheat up and the grain only folks are prepping grain drills or dealing with summer crop harvest. While this blog is focused on the wheat producers it should be acknowledged as a work of caution for anyone who pulls soil samples in environments where the temps during sampling can be above the mid 80’s.
Following some fantastic work by our Wild Cat Soil Fertility Counterparts (see blog) we dug into the same question of how does soil sample handling post collection impact the results. Dr. Ruiz-Diaz results on the impact of storage on nitrogen values was not surprising. But we wanted to go the next step and add in sample bag type.
Our Project
We collected a soil ground and homogenized. Placed in three types of bags Ziploc, Commercial (resin lined paper that is closed) and SWFAL bag (breathable material). Placed samples in a mini-van (Field) and brought a group onto campus (Office). With both sets of samples we had temperature loggers collecting hourly data. Every three days we collected four samples from each location determined moisture content and ran a full spectrum soil analysis including CO2 burst. The soil we used had a OM of 1.1% and soil pH of 6.1

Now let’s back up. Why was the KSU data not surprising. Well for those certified and honorary nitrogen ninjas we understand that the N-cycle processes of mineralization, immobilization, and nitrification are biological reactions which are significantly impacted by soil moisture and soil temperature. But the short and quick version is that the microbes that convert organic N to mineral N (NH4) and convert NH4 to NO3 like warm conditions with good soil moisture. So, when you pull soil samples, hopefully there is some moisture in the soil and then you place it in a bag and seal it. This creates an effective greenhouse environment where moisture cannot escape and is the perfect place for microbes to microbial things. Therefore, you expect the organic cycle and nitrogen cycle to move and move quickly through the processes of mineralization, ammonification, and nitrification.
Back to the data.
First, we have the average daily temperatures from the sitting vehicle and the lab. On days 2-5 the average temp of the van was over 100°, after that the temps dropped. One thing to remember is the temp of the van is an average of a wide-ranging highs and lows between morning and midday while the office temperature being stable throughout the entire day.

What is the most interesting and revealing data may be the soil moisture of the samples. Unfortunately, day 3 samples went straight to the oven, but you can see by day 6 the SFWAL bags (yellow line) had dried significantly while it wasn’t until day 27 did the Commercial bags in the van (Black line w/ triangle) show signs of drying. It’s also important to note that while there are two lines (Field and Office) for the Commercial bags and Ziploc bags, but only one for the SWFAL bags. That is because for all variables measured there was never a significant difference between the SWFAL Field value and the SWFAL Office value.

We are going to start off with the variable that changed the most, NO3-N. This is also the form of N that is measured in most soil test. With a starting point of 3.4 lbs. N ac-1 (based on a six inch soil sample depth) by day 6 the Field samples had jumped to 15.6 lbs. The office samples had much slower increase in NO3 with it taking 9 days to reach 10 lbs. You can also see that the error bars start showing up in the samples after about two weeks. The bars show the range in the sample results of a treatment. The interesting thing we saw was that the location within the van was significant. We set each replication of samples in a different spot in the van, the front seat, middle seat, and rear. The thermometers set with each replications showed that each area of the van had slightly differing temperature, which in turn affected the samples.

Ammonium (NH4-N), which is only reported by a few labs is also impacted by the storage location and bag type, however not to the sample level as NO3-N. Effectively the SWFAL bags and all samples taken to the office maintained consistent NH4 levels. The Com bag and Ziplocs left in the van however saw a significant increase in NH4 over time, basically a 2 lb per day increase.

Of the other measured parameters such as potassium, sulfur, and CO2 burst data were effected. These will be discussed more in depth in a peer reviewed publication and factsheet.
Take Home’s from the Work
While the majority of the nutrients were relatively un affected by the bag type or storage those that are more heavily influenced by organic matter and biological activity where, this includes many of the soil health parameters. The use of SWFAL soil bags resulted in data that was not impacted by storage or time. While most clients of the OSU lab use these bags, more than one samples been brought into the county via a sealed Ziploc baggie. However the majority of the soil samples collected are placed into the resin lined paper bags and sealed. This data set is also important to those who use laboratories that run the Haney test which includes measures of NO3, NH4, Organic N, CO2 respiration and H2O extracted Organic C. It is critical that when using these type of bags that the samples be brought into climate controlled facilities as soon as possible. Its good to remember when looking at the data that the soil we used had a OM of 1.1%, initial NO3 of 3.4 lbs. and NH4 of 14 lbs. A soil with a higher OM% could have even greater change. Another viable is if the soil sample depth is greater than 6”. If your sample depth is 8” then the value is 33% higher.
Questions or comments feel free to reach out via email or social media.
Brian Arnall, Precision Nutrient Management b.arnall@okstate.edu
Chinch bugs are active!
Both Josh Lofton and myself have been talking a lot about the magnitude of chinch bugs we’ve seen this year and the devastation they are having on the crops, both false and true chinch bugs. They have marched through sorghum and now are being found in corn fields. They seen especially bad in failed wheat fields. And in my fields anywhere I had a crabgrass. We are also hearing and seeing a significant increase in blister beetles and stink bugs in soybeans. As a soil scientist all I can recommend is to scout Often, and contact an entomologist or trusted advisor. Kansas State just put out and E-update yesterday with this article from Jeff Whitworth I wanted to share.
Chinch bugs are active in Kansas
Guest Author Jeff Whitworth, Extension Entomologist jwhitwor@ksu.edu
Chinch bugs have historically been a problem in Kansas–in lawns, golf courses, turf farms, etc. But in agriculture, they are mainly a problem in sorghum. However, they can also affect corn and occasionally wheat. Since they are true bugs, chinch bugs may attack any grass where they insert their mouthparts into the plants and suck out the juice. This often has little to no effect on the plant unless there are large numbers of bugs and/or the plants are growing under less-than-ideal conditions so that they are already stressed. Chinch bug feeding simply adds to this stress.
Sampling for chinch bugs the week of July 4 indicated that 95% of the chinch bug population in north central Kansas were adults (Figure 1). Adults don’t feed as much as nymphs but are more concerned with mating, oviposition, etc. This means the majority of feeding in crops (sorghum, corn, etc.) is still to come after the nymphs hatch (Figure 2).
Treating for chinch bugs needs to be accomplished using as much carrier (water) as practical to ensure the insecticide gets good coverage on the plants, including the base of the plants (sprays directed at the base of the plants will help). Nymphs produced now will most likely become adults in 3-4 weeks, then mate and start the process all over again for another generation, which will then move to fall-planted wheat, then on to overwintering sites. They overwinter in bunch grasses then move to wheat in the spring to deposit eggs and start all over again.


Original link https://eupdate.agronomy.ksu.edu/article_new/chinch-bugs-are-active-in-kansas-553-4
To Subscribe to KSU E-update. https://eupdate.agronomy.ksu.edu/index_new_prep.php




