Home » Soil pH and Soil Acidity
Category Archives: Soil pH and Soil Acidity
Laboratory evaluation of Liquid Calcium
Liquid calcium products have been around for a long time. The vast majority of these products are either a calcium chloride or chelated calcium base which is now commonly found with the addition of a humic acid, microbial, or micronutrient. Many of these make promises such as “raises your soil pH with natural, regenerative, liquid calcium fertilizers that correct soil pH quickly, efficiently, and affordably!”. From a soil chemistry aspect the promise of adding 3 to 5 gallons of a Ca solution, which is approximately 10% Ca, will raise the soil pH is impossible on a mass balance approach. In this I mean that to increase the pH of an acid soil {soil pH is the ratio of hydrogen (H) and hydroxide (OH) in the soil, and having an acid soil means the concentration of H is greater than that of OH} requires a significant portion of the H+ that is in solution and on soil particle to be converted to OH, or removed from the system entirely.
The blog below walks through the full chemical process of liming a soil but in essence to reduce the H+ concentration we add a cation (positively charged ion) such as Ca or magnesium (Mg) which will kick the H+ of the soil particle and a oxygen (O) donator such as CO2 with ag lime or (OH)2 which is in hydrated lime. Each of these O’s will react with two H’s to make water. And with that the pH increases.
However regardless of the chemistry, there is always a lot of discussion around the use of liquid calcium Therefore we decided to dig into the question with both field and laboratory testing. This blog will walk through the lab portion.
This was a laboratory incubation study. The objective was to evaluation the impact of the liquid Ca product (LiqCa**) on the soil pH, buffer capacity, Ca content and CEC of two acidic soils. LiqCa was applied at three rates to 500 g of soil. The three rates were equivalent to 2, 4, and 6 gallon per acre applied on a 6” acre furrow slice of soil. One none treated check and two comparative products were also applied. HydrateLime (CaO) as applied at rate of Ca equivalent to the amount of Ca applied via LiqCa, which was approximately 1.19 pounds of Ca per acre. Also AgLime (CaCO3) was applied at rates equivalent to 1, 2, and 4 ton effective calcium carbonate equivalency (ECCE). The Ag lime used in the study had a measured ECCE of 92%. The two soils selected for both acidic but had differing soil textures and buffering capacities. The first LCB, had an initial soil pH (1:1 H2O) of 5.3 and a texture of silty clay loam and Perkins had a initial pH of 5.8 and is a sandy loam texture. Both soils had been previously collected, dried, ground, and homogenized. In total 10 treatments were tested across two soils with four replications per treatment and soil.
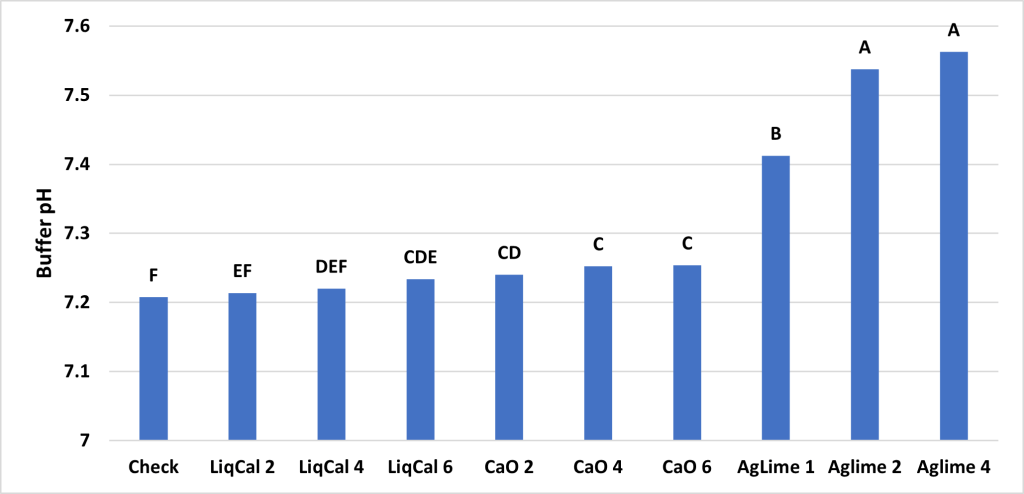
Project protocol, which has been used to determined site specific liming and acidification rates, was to apply the treatments to 500 grams of soil. Then for a period of eight weeks this soil wetted and mixed to a point of 50% field capacity once a week then allowed to airdry and be mixed again. At the initiation and every two weeks after soil pH was recorded from each treatment. The expectation is that soil pH levels will change as the liming products are impacting the system and at some point, the pH reaches equilibrium and no longer changes. In this soil that point was week six however the trail was continued to week eight for confirmation. See Figures 1 and 2.


ANOVA Main effect analysis showed that Soil was not a significant effect so therefore both soils were combined for further analysis. Figure 3 shows the final soil pH of the treatments with letters above bars representing significance between treatments. In this study all treatments were significantly greater than the check with exception of LiqCal 2 and CaO 6. Neither LiqCal or CaO treatments reached the pH level of Aglime, regardless of rate.

Summary
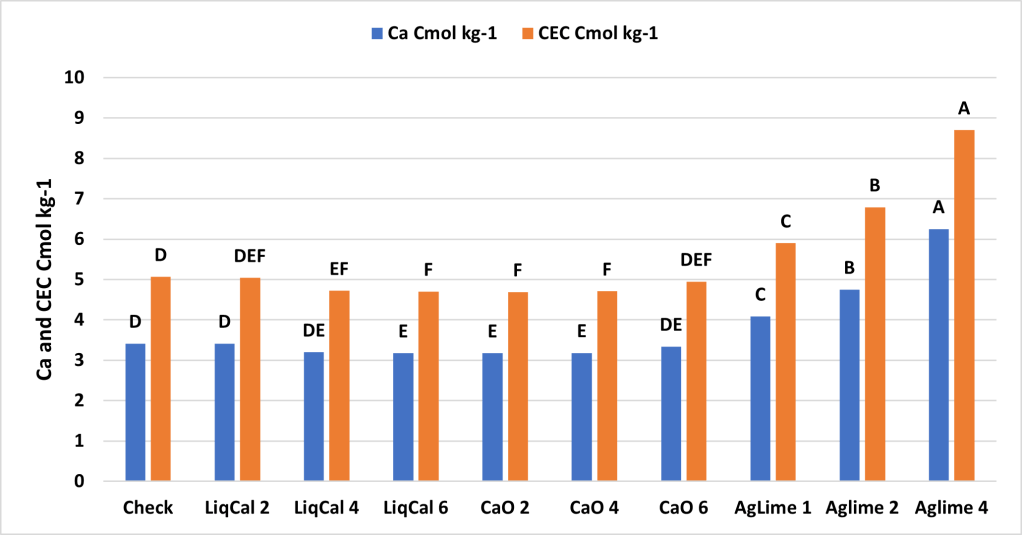
The incubation study showed that application of LiqCal at a rate of 4 and 6 gallons per acre did significantly increase the soil pH by 0.1 pH units and 6 gallons per acre increased the Buffer index above the check by 0.03 units. Showing the application of LiqCal did impact the soil. However the application of 1 ton of Ag lime resulted in significantly great increase in soil pH, 1.0 units by 8 weeks and a buffer index change of 0.2 units. The Aglime 1 was statistically greatly than all LiqCal treatments. Ag lime 2 and 4 were both statistically greater than Ag lime 1 with increasing N rate with increasing lime rate. Given the active ingredient listed in LiqCal is CaCl, this result is not unexpected. Ag lime changes pH by the function of CO3 reacting H+ in large quantities. In a unsupported effort a titration was performed on LiqCal, which show the solution was buffered against pH change. However it was estimated that a application of approximately 500 gallons per acre would be needed to sufficiently change the soil pH within a 0-6” zone of soil.
Results of the field study.
https://osunpk.com/2025/06/02/field-evaluation-of-lime-and-calcium-sources-impact-on-acidity/
Take Home
The application of a liquid calcium will add both calcium and chloride which are plant essential nutrients and can be deficient. In a soil or environment suffering from Cl deficiency specifically I would expect an agronomic response. However this study suggest there is no benefit to soil acidity or CEC with the application rates utilized (2, 4, and 6 gallon per acre).
** LiqCal The product evaluated was derived from calcium chloride. It should be noted that since the completion of the study this specific product used has changed its formulation to a calcium chelate. This change however would not be expected to change the results as the experiment did include a equivalent calcium rate of calcium oxide.
Other articles of Interest
https://extension.psu.edu/beware-of-liquid-calcium-products
https://foragefax.tamu.edu/liquid-calcium-a-substitute-for-what/
Any questions or comments feel free to contact me. b.arnall@okstate.edu
Field evaluation of lime and calcium sources impact on Acidity.
At the same time we initiated a lab study looking at the application of LiqCal https://osunpk.com/?p=2096 , we also initiated a field trial to look at the multi-year application of LiqCal, Pelletized Lime and Ag-Lime.
A field study was implemented on a bermudagrass hay meadow near Stillwater in the summer of 2019. The study looked to evaluate the impact of multiple liming / calcium sources impact on forage yield and soil properties. This report will focus on the impact of treatments on soil properties while a later report will discuss the forage results.
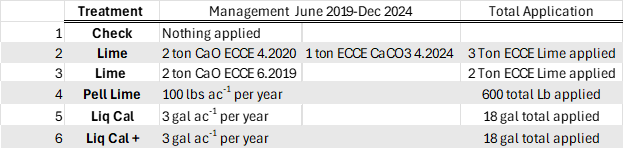
Table 1. has the management of the six treatments we evaluated, all plots had 30 gallons of 28-0-0 streamed on each spring in May. Treatment 1 was the un-treated check. Treatment 2, was meant to be a 2 ton ECCE (Effective Calcium Carbonate Equivalency) Ag Lime application when we first implemented the plots in 2019, but we could not source any in time so we applied 2.0 ton ECCE hydrated lime (CaO) the next spring. The spring 2023 soil samples showed the pH to have fallen below 5.8 so and Ag lime was sourced from a local quarry and 1.0 ton ECCE was applied May 2024. Treatment 3, was meant to complement Treatment 2 as an additional lime source of hydrated lime, it was applied June 2019. My project has used hydrated lime as a source for many years as it is fast acting and works great for research. Treatment 4 had 100 lbs. of pelletized lime applied each spring. The 100 lbs. rate was based upon recommendation from a local group that sells Pell lime. Treatments 5 and 6 were two liquid calcium products *Liq Cal * and **Lig Cal+ from the same company. The difference based upon information shared by the company was the addition of humic acid in the Liq Cal+ product. Both LiqCal and LiqCal+ where applied at a rate of 3 gallons per acre per year, with 17 gallons per acre of water as a carrier. Table 1, also shows total application over the six years of the study.
After six years of applications and harvest it was decided to terminate the study. The forage results were intriguing however little differences where seen in total harvest over the six years, highlighting a scenario I have encountered in the past on older stands of bermuda. That data will be shared in a separate blog.
The soils data however showed exceptionally consistent results.
In February of 2025 soil samples were collected from each plot at depths of 0-3 inch’s and 0-6 inches (Table 2.). It was our interest to see if the soil was being impacted below the zone we would expect lime and calcium to move without tillage, which if 0-3″. Figure 1. below shows the soil pH of the treatments at each depth. In the surface (blue) the Ag Lime and Hydrated lime treatments both significantly increased from 4.78 to 6.13 and 5.7 respectively. While the Pel lime, LiqCal and LiqCal+ had statistically similar pH’s as the check at 4.8, 4.65, and 4.65. It is important to note that the Ag Lime applied in May of 2024 resulted in a significant increase in pH from the 2019 application of Treatment 3. The Spring of 2024 soil samples showed that the two treatments ( 2 and 3 ) were equivalent. So within one year of application the Ag lime significantly raised soil pH.
As expected the impact on the 3-6″ soil pH was less than the surface. However, the Ag Lime and Hydrated lime treatments significantly increased the pH by approximately 0.50 pH units. This is important data as the majority of the literature suggestion limited impact of lime on the soil below the 3″ depth.

The buffer pH of a soil is used to determine the amount of lime needed to change the soils pH. In Figure 2. while numeric differences can be seen, no treatment statistically impacted the buffer pH at any soil depth.

The soil calcium level was also measured. As with 0-3″ pH and Buffer pH the Ag Lime and Hydrated lime had the greatest change from the check. These treatments were not statistically greater than the Pell Lime but where higher than the LiqCal and LiqCal+.
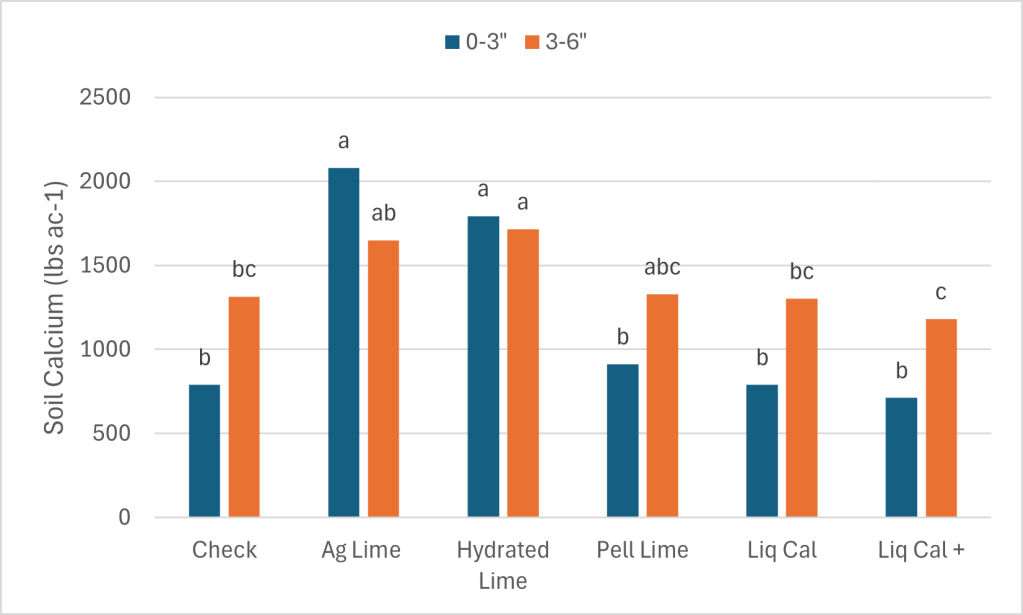

Each value is the average of four replicates.
Take Homes
In terms of changing the soils pH or calcium concentration, as explained in the blog https://osunpk.com/2023/01/24/mechanics-of-soil-fertility-the-hows-and-whys-of-the-things/, it takes a significant addition of cations and oxygens to have an impact. This data shows that after six years of continued application of pelletized lime and two liquid calcium products the soil pH did not change. While the application of 2 ton ECCE hydrate lime did.
Also within one year of application Ag lime the soil pH significantly increased.
* LiqCal The product evaluated was derived from calcium chloride. It should be noted that since the completion of the study this specific product used has changed its formulation to a calcium chelate. This change however would not be expected to change the results as the experiment did include a equivalent calcium rate of calcium oxide.
** LiqCal+ The product evaluated was derived from calcium chloride. It should be noted that since the completion of the study this specific product used has changed its formulation. The base was changed from calcium chloride to a calcium chelate. Neither existing label showed Humic Acid as a additive, however the new label has a a list of nutrients at or below 0.02% (Mg, Zn, S, Mn, Cu, B, Fe) and Na at .032% and is advertised as having microbial enhancements.
Any questions or comments feel free to contact me. b.arnall@okstate.edu
Phosphorus decisions, Is it worth cutting P?
With the current conditions and input cost many wheat producers are considering cutting back on inputs. I can’t disagree with the plan, but I would caution against what you cut. If you have read any of my past blogs, or seen me speak, you should know I’m all for cutting back on pre-plant nitrogen (N). Based on some recent trials I would not argue cutting the potassium (K) side, but phosphorus (P) that’s another story that we will walk through in this blog.
First and foremost, soil testing is the key to P management. If your soil test is below the critical threshold for the test you use, 32.5 for Mehlich 3 (M3P), then you need to add phos. We have enough work that shows current recommendations work for P in wheat. Reeds paper Evaluation of incorporated phosphorus fertilizer recommendations on no-till managed winter wheat Link to Paper goes over soil test recommendations in no-till and the recent double crop soybean project Double Crop P and K Blog highlights the importance of P fertility, regardless of yield level. Also if your soil test is below a 5.5 and you haven’t limed (Liming is the best solution, Band-aids not so cheap Blog ), then the next best option is adding additional P to alleviate the aluminum toxicity Band-aids for low pH Blog. In-short if the fields soil test P and or pH is below optimum you should not forgo P application.
But the primary reason I am writing this blog is for those looking at fields with composite soil test that is right around the critical thresholds, and they are trying to make the call on to apply P or not to apply P. Even on fields with soil test values in the good level, I am usually in favor of banding in-furrow fertilizer wheat, but not because of the same reasons I am for corn. With corn you are planting in cool soils and the availability of nutrients like P is lower in cool wet soils. For wheat cold soil isn’t the concern until we reach the end of the planting window. It will serve as a bit of a “pop-up” as the crop comes out of dormancy in the spring. I have also seen little to no value of N applied in furrow. I see same response to DAP (18-46-0), MAP (11-52-0), and TSP (0-46-0) when all applied at same rate of P. Meaning it was the P not N making the difference.
For me the reason I still recommend getting a little phosphate out even when the soil test comes back is that the great majority of fields have a large range of variability. Looking at a set of 650 grid sampled fields across Oklahoma and Kansas it showed on average soil pH 6.0 and M3P was 34 ppm. Both pH and P are at adequate/optimum levels. However, the average is usually somewhere between the low and high point and in this data set and the range of soil pH was 1.8 units and the range in M3P was 67 ppm. That meant on average of the 648 field with pH values the average difference between low pH and high pH was 1.8 units and the difference between low P and high P was 64 ppm.
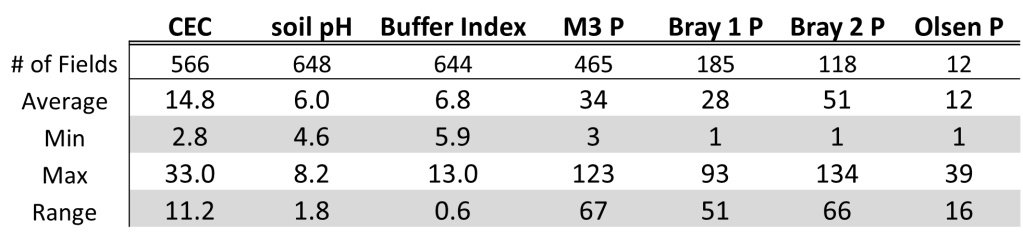
The field below is from Kingfisher county and was sampled at a resolution of 10 acres per sample. This is a fairly course resolution for grid sampling but provides a great view of how variable our soils can be. The field average pH is 5.3, which is below optimum but our aluminum tolerant wheats would be able to handle fairly well. For the P the average is 22 ppm which needs about 18 lbs of P2O5 to max yields. If the farmer applied a flat rate of 20 lbs there would be significant forage loss on about 65% of the field, for grain only about 45% of the field due to underapplication of P. Note that low P and low pH are not correlated well, meaning the areas low in pH are not always low in P.

Banding P makes it more efficient because it slows the rate of tie. However, we have plenty data that says broadcast applied P is still a great option, even after planting. So what are my take homes from this blog?
First: If you are grazing wheat get down 40-50 lbs of N pre. But I have plenty of data the pre-plant N on grain only wheat is not needed. I have the same amount of data that shows the only value of in-furrow N for grain only is that it forces you to plant more seeds, because it just lowers stand.
Second: When it comes to wheat pay attention to Phosphorus and soil pH. Even our acid tolerant wheats preform better in neutral soil pHs, especially forage wise.
Third: A composite soil sample is an AVERAGE of the field. If your average is right at the ok level (pH of 5.6ish and M3P of 30 ppm), then half of your field is below optimum and will benefit from P.
Fourth: If you can band P great, but if you cant broadcast is still a viable option. Do Not Skip P when soil test says there is a need.
Questions or comments please feel free to reach out.
Brian Arnall b.arnall@okstate.edu
Banding P as a band-aid for soil acidity, not so cheap now.
Whoi Cho, PhD student Ag Economics advised by Dr. Wade Brorsen
Raedan Sharry, PhD Student Soil Science advised by Dr. Brian Arnall
Brian Arnall, Precision Nutrient Management Extension.
In 2014 I wrote the blog Banding P as a Band-Aid for low-pH soils. Banding phosphate to alleviate soil acidity has been a long practiced approach in the southern Great Plains. The blog that follows is a summary of a recent publication that re-evaluated this practices economic viability.
Many Oklahoma wheat fields are impacted by soil acidity and the associated aluminum (Al) toxicity that comes with the low soil pH. The increased availability of the toxic AL3+ leads to reduced grain and forage yields by impacting the ability of the plant to reach important nutrients and moisture by inhibiting root growth. Aluminum can also tie up phosphorus in the soil, further intensifying the negative effects of soil acidity. More on the causes and implication of soil acidity can be found in factsheet PSS-2239 or here (https://extension.okstate.edu/fact-sheets/cause-and-effects-of-soil-acidity.html). The acidification of many of Oklahoma’s fields has left producers with important choices on how to best manage their fields to maximize profit.
Two specific management strategies are widely utilized in Oklahoma to counter the negative impacts of soil acidification: Lime application and banding phosphorus (P) fertilizer with seed. While banding P with seed ties up Al allowing the crop to grow, this effect is only temporary, and application will be required every year. The effects of liming are longer lasting and corrects soil acidity instead of just relieving Al toxicity. Historically banding P has been a popular alternative to liming largely due to the much lower initial cost of application. However, as P fertilizers continue to increase in cost the choice between banding P and liming needed to be reconsidered.
A recent study by Cho et al.,2020 compared the profitability of liming versus banding P in a continuous wheat system considering the impacts that lime cost, wheat price and yield goal has on the comparison. This work compared the net present value (NPV) of lime and banded P. The study considered yield goal level (40 and 60 bu/ac) as well as the price of P2O5 fertilizer and Ag Lime. The price of P2O5 used in this study was $0.43 lb-1 while lime price was dictated by distance from quarry, close to quarry being approximately $43 ton-1 and far being $81 ton-1. For all intents and purposes these lime values are equivalent to total lime cost including application. Wheat prices utilized in the study were $5.10 bu-1 and $7.91 bu-1. It is important to note that baseline yield level was not considered sustainable under banded P management in this analysis. This resulted in a decrease in yield of approximately 3.2 bu ac-1 per year. This is attributable to the expected continued decline in pH when banding P is the management technique of choice.
The analysis in this work showed that lime application is cost prohibitive in the short term (1 year) when compared with banding P regardless of lime cost, yield goal level, and wheat value (within the scope of this study). This same result can be seen over a two-year span when yield is at the lower level (40 bu ac-1). While in the short-term banding P was shown to be a viable alternative to liming, as producers are able to control ground longer lime application becomes the more appealing option, especially when producers can plan for more than 3 years of future production. In fact, under no set of circumstances did banding P provide greater economic return than liming regardless of crop value, yield, or liming cost when more than 3 years of production were considered and only under one scenario did banded P provide a higher NPV in a 3-year planning horizon.
While historically banding P was a profitable alternative to lime application for many wheat producers the situation has likely drastically changed. At the time of writing this blog (09/17/2021) Diammonium Phosphate (DAP) at the Two Rivers Cooperative was priced at $0.78 lb-1. of P2O5. This is a drastic increase in P cost over the last year or so since Cho et al. was published in 2020. With P fertilizer prices remaining high it will be important for producers to continue to consider the value of liming compared to banded P. This is particularly crucial for those producers who can make plans over a longer time frame, especially those more than 3 years.
Addendum: As fertilizer prices have continued to rise a quick analysis utilizing the $0.78 lb-1 of P2O5was completed to consider the higher P fertilizer cost. Under this analysis an estimated decrease in NPV of approximately $38 an acre for P banding occurred. When considering this change in NPV, lime application becomes the more profitable option for alleviation of soil acidity symptoms even in the short term (assuming lime price values are equivalent to the previous analysis). This underlines the fact that it is imperative to consider the impact on profitability of the liming vs. banding P decision in the current economic climate for agricultural inputs.
Link to the Open Access Peer Reviewed publication “Banding of phosphorus as an alternative to lime for wheat in acid soil” https://doi.org/10.1002/agg2.20071
Soil Acidity and Cotton Production
Raedan Sharry, Precision Nutrient Management Ph.D. student
Brian Arnall, Precision Nutrient Management Extension Specialist
Cotton production in Oklahoma has expanded the past decade into areas which other production systems such as continuous wheat may have traditionally dominated. Fields that have been managed for continuous wheat production may have become acidic in response to management practices, such as the use of ammoniacal nitrogen fertilizers. In response to the acidification of these soils it may be important to recognize and understand the potential impacts of soil acidification on cotton production.
To better document the impact of soil pH on cotton lint yield and quality a study was conducted over the 2019 and 2020 growing seasons at two locations: Stillwater, OK (EFAW farm) and Perkins, OK. Soil pH in these experiments were adjusted to a depth of approximately 6 inches using aluminum sulfate (acidifying) and hydrated lime (alkalinizing). All three locations were planted to two varieties; NexGen 3930 and Deltapine 1612 at a rate of approximately 35,000 seeds per acre, into plots with soils ranging in pH from 4.0 to 8.0.
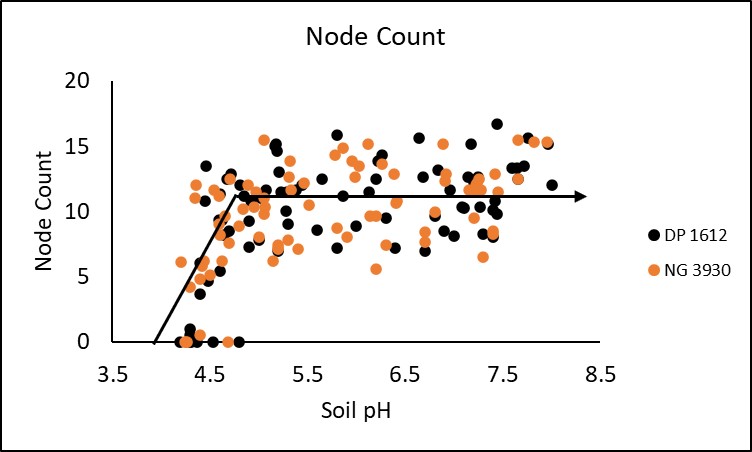
In season measurements taken included stand count, plant height, node count and NDVI (normalized difference vegetative index. All four in season measurements demonstrated a significant critical threshold in which soil pH negatively impacted crop performance. Stand was significantly decreased at a soil pH of 5.3 or lower. This trend is displayed in Figure 1. Plant height and node count depicted in figures 2 and 3 respectively were both significantly decreased when soil pH dropped below 5.3 and 4.9, while NDVI began to deteriorate around a pH of 5.1 (Figure 4). Response to soil pH level was also visually observable as shown by Figure 5 from the Perkins 2019 location.




Yield levels across this experiment ranged from 0 to 1284 lbs. of lint per acre. In this work yield is reported as relative yield. To calculate relative yield the yield of each plot is normalized to the average of the three highest yields for that site. This method of reporting yield response allows this work to be applied across a range of yield environments.

When all sites and cultivars were combined relative yield reached a plateau at approximately 73% of yield with a critical threshold observed at a pH of 5.4. Below this critical threshold yield decreased at a rate of 37% per point of pH decline. This equates to 15% yield loss at a pH of 5.0, 33% yield loss at a pH of 4.5, and 52% yield loss at a pH of 4.5. This relationship is depicted in Figure 6. It is important to note that yield was 0 when pH was 4.3 in two plots. This represents the possibility for total crop failure when planting into very acidic conditions.
Differences in relative yield between cultivars were insignificant. However, further investigation may produce a significant difference. Critical threshold for the DP 1612 and NG 3930 cultivars were 6.1 and 5.2 respectively. This suggests that there may be value in further examining the influence of genetics on cotton response to soil acidity.

SUMMARY
The influence of soil pH level on cotton productivity is confirmed by this study. All three sites evaluated provided a strong correlation between soil pH and lint yield, as well as the in-season growth parameters measured. While this study is likely to be expanded to another location to provide a more robust evaluation of the potential impact of soil acidity on cotton, the current dataset provides ample evidence to conclude that soil acidity is likely to be detrimental to cotton production in the southern plains. Soil pH levels below 5.5 appear to provide the greatest opportunity for yield loss as depicted above. Lint quality measurements taken in the study (micronaire, length, uniformity, and strength) showed no consistent trends in the relationship between quality parameters and soil pH suggesting that soil acidity had a limited influence on lint quality characteristics. Cotton response to soil pH is likely to be influenced by the environment of a specific location and growing season. This underlines the importance of understanding the soil properties that negatively impact productivity, such as the presence of toxic forms of aluminum or manganese. It is also important to highlight the possibility of acidic conditions significantly affecting the ability of the crop to access important nutrients such as phosphorus. Under acidic conditions cotton productivity is likely to be significantly decreased unless soils are neutralized using a soil amendment such as lime.
Project Supported by the
Oklahoma Cotton Council
Cotton Incorporated
Oklahoma Fertilizer Checkoff Program

