Home » Posts tagged 'calcium'
Tag Archives: calcium
Field evaluation of lime and calcium sources impact on Acidity.
At the same time we initiated a lab study looking at the application of LiqCal https://osunpk.com/?p=2096 , we also initiated a field trial to look at the multi-year application of LiqCal, Pelletized Lime and Ag-Lime.
A field study was implemented on a bermudagrass hay meadow near Stillwater in the summer of 2019. The study looked to evaluate the impact of multiple liming / calcium sources impact on forage yield and soil properties. This report will focus on the impact of treatments on soil properties while a later report will discuss the forage results.
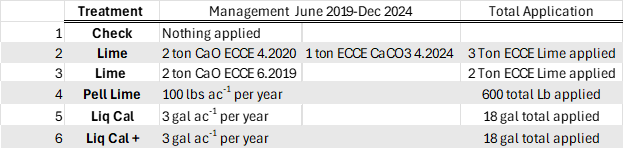
Table 1. has the management of the six treatments we evaluated, all plots had 30 gallons of 28-0-0 streamed on each spring in May. Treatment 1 was the un-treated check. Treatment 2, was meant to be a 2 ton ECCE (Effective Calcium Carbonate Equivalency) Ag Lime application when we first implemented the plots in 2019, but we could not source any in time so we applied 2.0 ton ECCE hydrated lime (CaO) the next spring. The spring 2023 soil samples showed the pH to have fallen below 5.8 so and Ag lime was sourced from a local quarry and 1.0 ton ECCE was applied May 2024. Treatment 3, was meant to complement Treatment 2 as an additional lime source of hydrated lime, it was applied June 2019. My project has used hydrated lime as a source for many years as it is fast acting and works great for research. Treatment 4 had 100 lbs. of pelletized lime applied each spring. The 100 lbs. rate was based upon recommendation from a local group that sells Pell lime. Treatments 5 and 6 were two liquid calcium products *Liq Cal * and **Lig Cal+ from the same company. The difference based upon information shared by the company was the addition of humic acid in the Liq Cal+ product. Both LiqCal and LiqCal+ where applied at a rate of 3 gallons per acre per year, with 17 gallons per acre of water as a carrier. Table 1, also shows total application over the six years of the study.
After six years of applications and harvest it was decided to terminate the study. The forage results were intriguing however little differences where seen in total harvest over the six years, highlighting a scenario I have encountered in the past on older stands of bermuda. That data will be shared in a separate blog.
The soils data however showed exceptionally consistent results.
In February of 2025 soil samples were collected from each plot at depths of 0-3 inch’s and 0-6 inches (Table 2.). It was our interest to see if the soil was being impacted below the zone we would expect lime and calcium to move without tillage, which if 0-3″. Figure 1. below shows the soil pH of the treatments at each depth. In the surface (blue) the Ag Lime and Hydrated lime treatments both significantly increased from 4.78 to 6.13 and 5.7 respectively. While the Pel lime, LiqCal and LiqCal+ had statistically similar pH’s as the check at 4.8, 4.65, and 4.65. It is important to note that the Ag Lime applied in May of 2024 resulted in a significant increase in pH from the 2019 application of Treatment 3. The Spring of 2024 soil samples showed that the two treatments ( 2 and 3 ) were equivalent. So within one year of application the Ag lime significantly raised soil pH.
As expected the impact on the 3-6″ soil pH was less than the surface. However, the Ag Lime and Hydrated lime treatments significantly increased the pH by approximately 0.50 pH units. This is important data as the majority of the literature suggestion limited impact of lime on the soil below the 3″ depth.

The buffer pH of a soil is used to determine the amount of lime needed to change the soils pH. In Figure 2. while numeric differences can be seen, no treatment statistically impacted the buffer pH at any soil depth.

The soil calcium level was also measured. As with 0-3″ pH and Buffer pH the Ag Lime and Hydrated lime had the greatest change from the check. These treatments were not statistically greater than the Pell Lime but where higher than the LiqCal and LiqCal+.
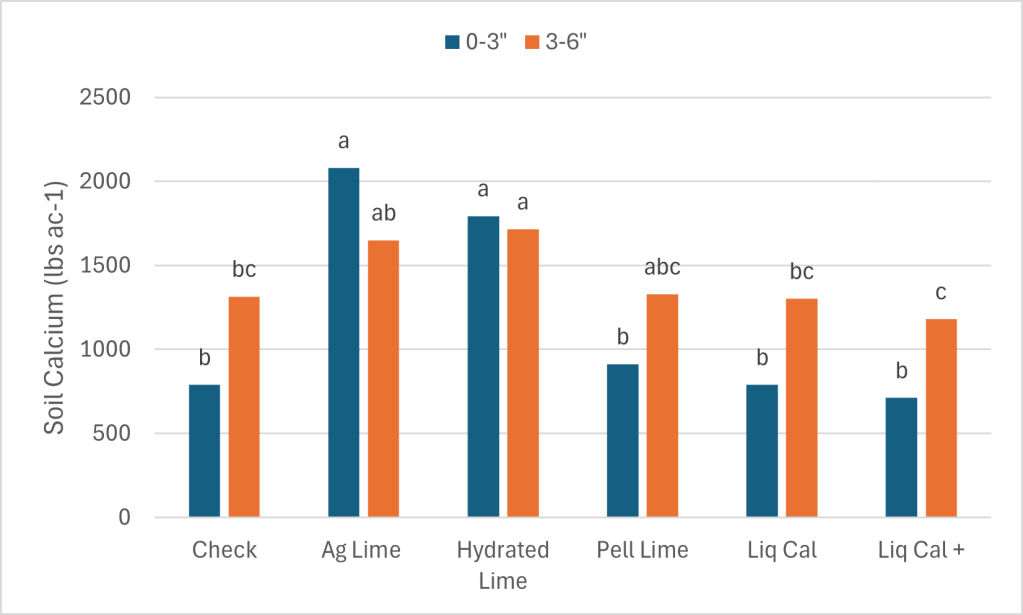

Each value is the average of four replicates.
Take Homes
In terms of changing the soils pH or calcium concentration, as explained in the blog https://osunpk.com/2023/01/24/mechanics-of-soil-fertility-the-hows-and-whys-of-the-things/, it takes a significant addition of cations and oxygens to have an impact. This data shows that after six years of continued application of pelletized lime and two liquid calcium products the soil pH did not change. While the application of 2 ton ECCE hydrate lime did.
Also within one year of application Ag lime the soil pH significantly increased.
* LiqCal The product evaluated was derived from calcium chloride. It should be noted that since the completion of the study this specific product used has changed its formulation to a calcium chelate. This change however would not be expected to change the results as the experiment did include a equivalent calcium rate of calcium oxide.
** LiqCal+ The product evaluated was derived from calcium chloride. It should be noted that since the completion of the study this specific product used has changed its formulation. The base was changed from calcium chloride to a calcium chelate. Neither existing label showed Humic Acid as a additive, however the new label has a a list of nutrients at or below 0.02% (Mg, Zn, S, Mn, Cu, B, Fe) and Na at .032% and is advertised as having microbial enhancements.
Any questions or comments feel free to contact me. b.arnall@okstate.edu
Soil calcium and magnesium levels: Does the ratio make a difference?
Guest Author
Dorivar Ruiz-Diaz,
Nutrient Management Specialist
Kansas State University
Is it important to have the proper ratio of calcium (Ca) and magnesium (Mg) in the soil? Producers may ask this question as they have their soil tested for nutrient levels in the summer before wheat planting begins. This question may also arise at the moment of lime purchase, which can be an important source of Ca and Mg.
Calcium and Mg are plant-essential nutrients. All soils contain Ca and Mg in the form of cations (positively charged ions, Ca++ and Mg++) that attach to the soil clay and organic matter; these are also the forms taken up by crops. The relative proportion of these elements, as well as the total amount in the soil, depends mainly on the soil parent material. In Kansas soils, the levels of Ca and Mg are typically high and crop deficiencies are rare.
Soils typically have higher Ca levels than Mg. Table 1 gives the amount and ratios of Ca and Mg for some soils in Kansas. Both nutrients are present in large quantities. Unusual cases of Ca or Mg deficiencies may be found in areas of very sandy soils.
| Table 1. Calcium, magnesium, and Ca:Mg ratio for several Kansas soils | |||
| Ca | Mg | Ca:Mg ratio | |
| Soil | cmol/kg | ||
| Canadian-Waldeck | 42 | 11 | 3.7 |
| Carwile | 22 | 4 | 5.2 |
| Chase | 198 | 30 | 6.7 |
| Crete | 111 | 29 | 3.8 |
| Harney | 202 | 15 | 13.2 |
| Harney-Uly | 200 | 12 | 16.1 |
| Keith | 127 | 38 | 3.3 |
| Las | 176 | 37 | 4.8 |
| McCook | 35 | 8 | 4.5 |
| Onawa | 163 | 28 | 5.8 |
| Ortello | 19 | 6 | 3.3 |
| Parsons | 80 | 23 | 3.5 |
| Tully | 158 | 38 | 4.2 |
Why would the ratio of Ca to Mg be important? The concept of an optimum Ca:Mg ratio started in the 1940s under the “basic cation saturation ratio” theory. The theory is that an “ideal soil” will have a balanced ratio of Ca, Mg, and potassium (K). According to this theory, fertilization should be based on the soil’s needs rather than crop’s needs — focusing on the ratio of crop nutrients present in the soil. This concept of an ideal Ca:Mg ratio has been debated by agronomists over the years. The suggested ideal ratio according to the theory is between 3.5 and 6.0, but this has never proven to be of significance.
There is very little research evidence to support any effect, either positive or negative, of the soil Ca:Mg ratio on crop production and yield. What research studies have been conducted in the laboratory and in the field show no effect of Ca:Mg ratio on crop yield. Despite this, the promotion of the ratio concept persists today. Furthermore, the initial work that derived this concept did not differentiate between crop response (alfalfa) due to the change in Ca:Mg ratio and the improvement in soil pH from lime application. It is reasonable to conclude that crop response can be expected from changes in soil pH rather than any change in the ratio of Ca:Mg.
One example of research conducted on this topic over the years is shown in Table 2. In that experiment, McLean and coworkers demonstrated the lack of relationship between Ca:Mg ratio and crop yield for several crops. The range of Ca:Mg ratios observed for the highest yields were not different from those observed for the lowest yields. The conclusion from that study was that to achieve maximum crop yield, attention should center on providing sufficient levels of these nutrients rather than attempting to find an adequate ratio. Therefore when these nutrients are present in optimum levels for plant growth, the relative ratio in the soil seems irrelevant.
| Table 2. Ratio of Ca:Mg for five crop-years comparing the highest and lowest yields obtained | ||||||
| Corn | Corn | Soybean | Wheat | Alfalfa | Alfalfa | |
| Yield level | Ca;Mg ratio | |||||
| Highest five | 5.7 – 26.8 | 5.7 – 14.2 | 5.7 – 24.9 | 5.7 – 14.0 | 5.7 – 26.8 | 6.8 – 26.8 |
| Lowest five | 5.8 – 21.5 | 5.0 – 16.1 | 2.3 – 16.1 | 6.8 – 21.5 | 8.2 – 21.5 | 5.7 – 21.5 |
Adapted from: McLean, E.O., R.C. Hartwig, D.J. Eckert, and G.B. Triplett. 1983. Basic cation saturation ratios as a basis for fertilizing and liming agronomic crops. II. Field studies. Agronomy Journal 75: 635-639.Ada – 21.veeio of Ca:Mg for five crop-years comparing the highest and lowest yields obtainedto the diseaseeo produced by Dan Don
In conclusion, trying to manage the ratio of Ca:Mg should not be used for a nutrient application or liming program. The center of attention should be to ensure that levels of Ca and Mg in the soil will not limit optimum plant growth. The relative concentration of Ca and Mg in commercial ag lime can be highly variable, and application should be based on the effective calcium carbonate (ECC) to achieve a target soil pH.
Dorivar Ruiz-Diaz, Nutrient Management Specialist
Kansas State University
ruizdiaz@ksu.edu